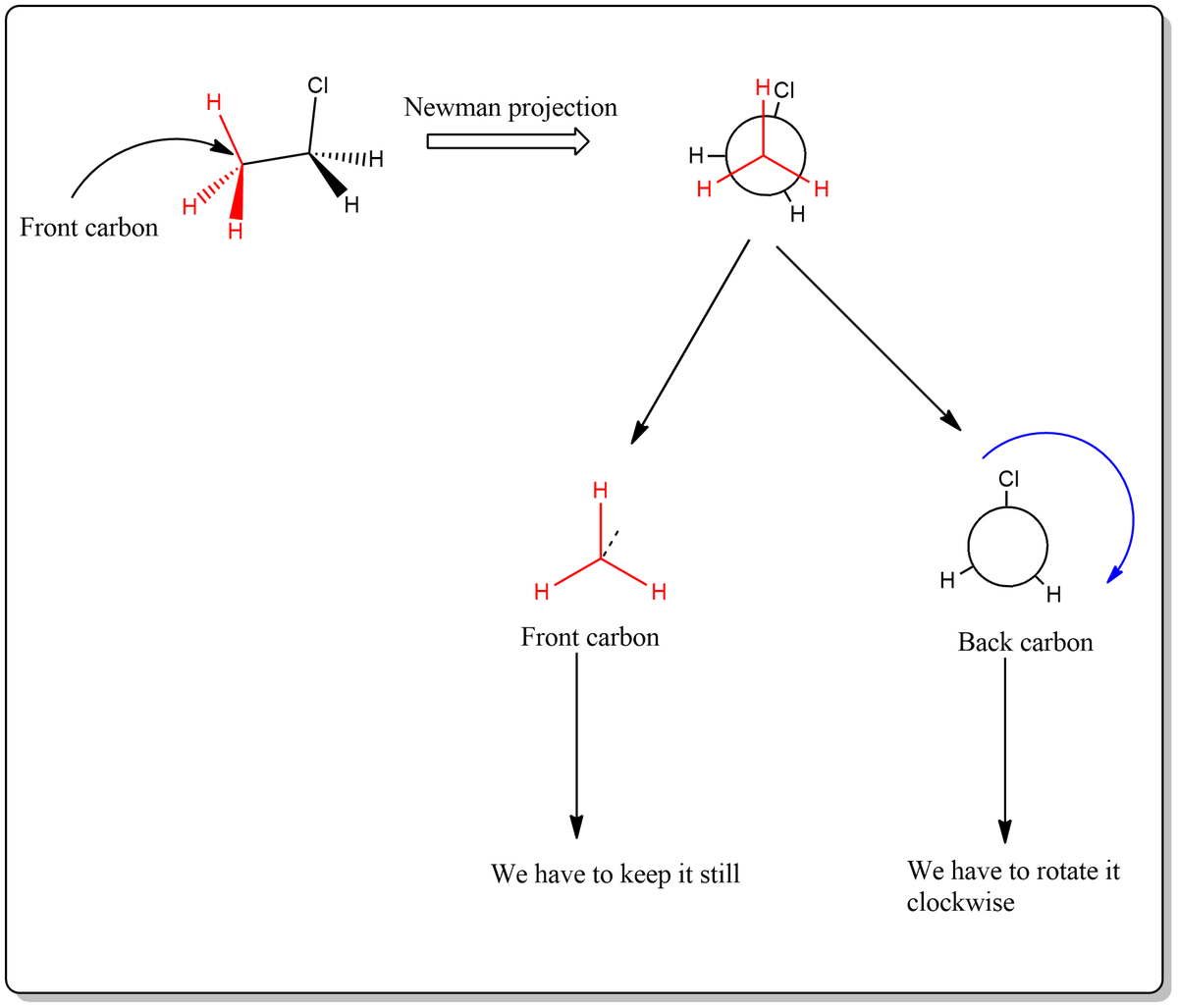
Match the conformers in Figure 5-9 to the position on the conformational analysis graph. Assume that you hold the front carbon (point) still and rotate the back carbon (circle) in a clockwise direction.
Reactive Intermediates
In chemistry, reactive intermediates are termed as short-lived, highly reactive atoms with high energy. They rapidly transform into stable particles during a chemical reaction. In specific cases, by means of matrix isolation and at low-temperature reactive intermediates can be isolated.
Hydride Shift
A hydride shift is a rearrangement of a hydrogen atom in a carbocation that occurs to make the molecule more stable. In organic chemistry, rearrangement of the carbocation is very easily seen. This rearrangement can be because of the movement of a carbocation to attain stability in the compound. Such structural reorganization movement is called a shift within molecules. After the shifting of carbocation over the different carbon then they form structural isomers of the previous existing molecule.
Vinylic Carbocation
A carbocation where the positive charge is on the alkene carbon is known as the vinyl carbocation or vinyl cation. The empirical formula for vinyl cation is C2H3+. In the vinyl carbocation, the positive charge is on the carbon atom with the double bond therefore it is sp hybridized. It is known to be a part of various reactions, for example, electrophilic addition of alkynes and solvolysis as well. It plays the role of a reactive intermediate in these reactions.
Cycloheptatrienyl Cation
It is an aromatic carbocation having a general formula, [C7 H7]+. It is also known as the aromatic tropylium ion. Its name is derived from the molecule tropine, which is a seven membered carbon atom ring. Cycloheptatriene or tropylidene was first synthesized from tropine.
Stability of Vinyl Carbocation
Carbocations are positively charged carbon atoms. It is also known as a carbonium ion.
![Match the conformers in Figure 5-9 to the position on the
conformational analysis graph. Assume that you hold the front
carbon (point) still and rotate the back carbon (circle) in a
clockwise direction.
A
B
D
E
F
[Choose ]
300 degrees
60 degrees
120 degrees
180 degrees
240 degrees
0 degrees
[Choose ]
[Choose ]
[Choose ]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F03317af0-4144-4b0a-a13c-c65183a22c1d%2F81b64985-1db5-409e-8100-21072bd2cd04%2Fzp6k46_processed.png&w=3840&q=75)

Step by step
Solved in 3 steps with 2 images









