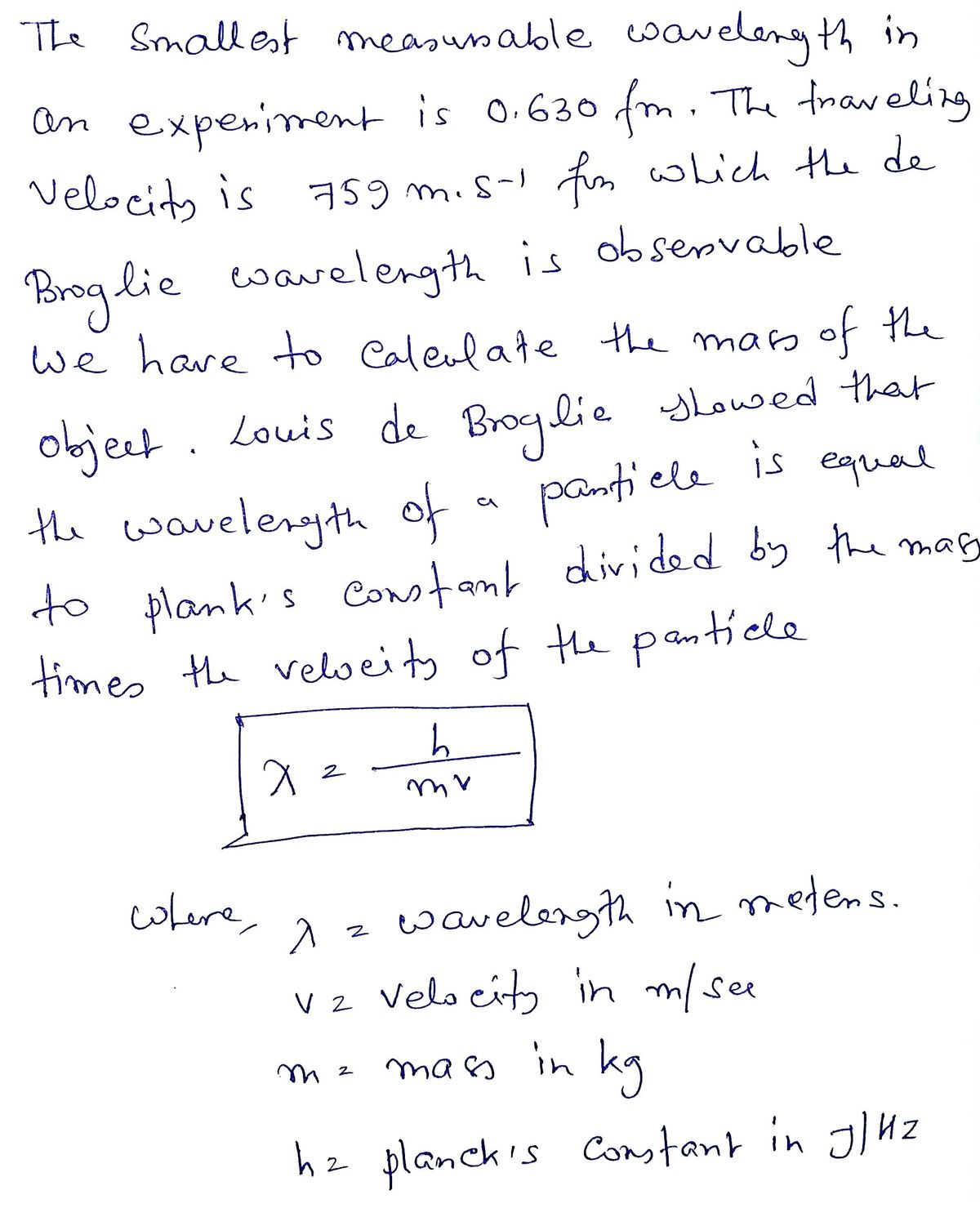
man Learnin Assuming that the smallest measurable wavelength in an experiment is 0.630 fm, what is the maximum mass of an object traveling at 759 m s for which the de Broglie wavelength is observable?
man Learnin Assuming that the smallest measurable wavelength in an experiment is 0.630 fm, what is the maximum mass of an object traveling at 759 m s for which the de Broglie wavelength is observable?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
How do I do this

Transcribed Image Text:o-6556-467c-aaOe-4b802c790a78/assi... D:
E Due: Fri, Dec 3
O Assignment Score:
11.4%
Resources
Give Up?
O Hint
Check Answer
Question 14 of 35
General Chemistry 4th Edition
McQuarrie Rock Gallogly
University Science Books
presented by Macmillan Learning
Assuming that the smallest measurable wavelength in an experiment is 0.630 fm, what is the maximum mass of an object
traveling at 759 m - s for which the de Broglie wavelength is observable?
m =
kg
Question Source: McQuarrie, Rock, And Gallogly 4e- General Chemsitry Publisher: University Science Books
1:13 PM
59°F Sunny ^ Cra )
10/12/2021
ASUS ZenBook
F12
IA
Insert
సాజలం
F11
Prt Sc
F10
F9
F8
F7
F4
F5
F6
F3
Bockspook
&
3
0
8.
5
%24
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY