Draw all resonance structures for each compound, and use the resonance structures to determine if the substituent has an electron-donating or electron-withdrawing resonance effect.

a) The resonating structures of Compound A are,
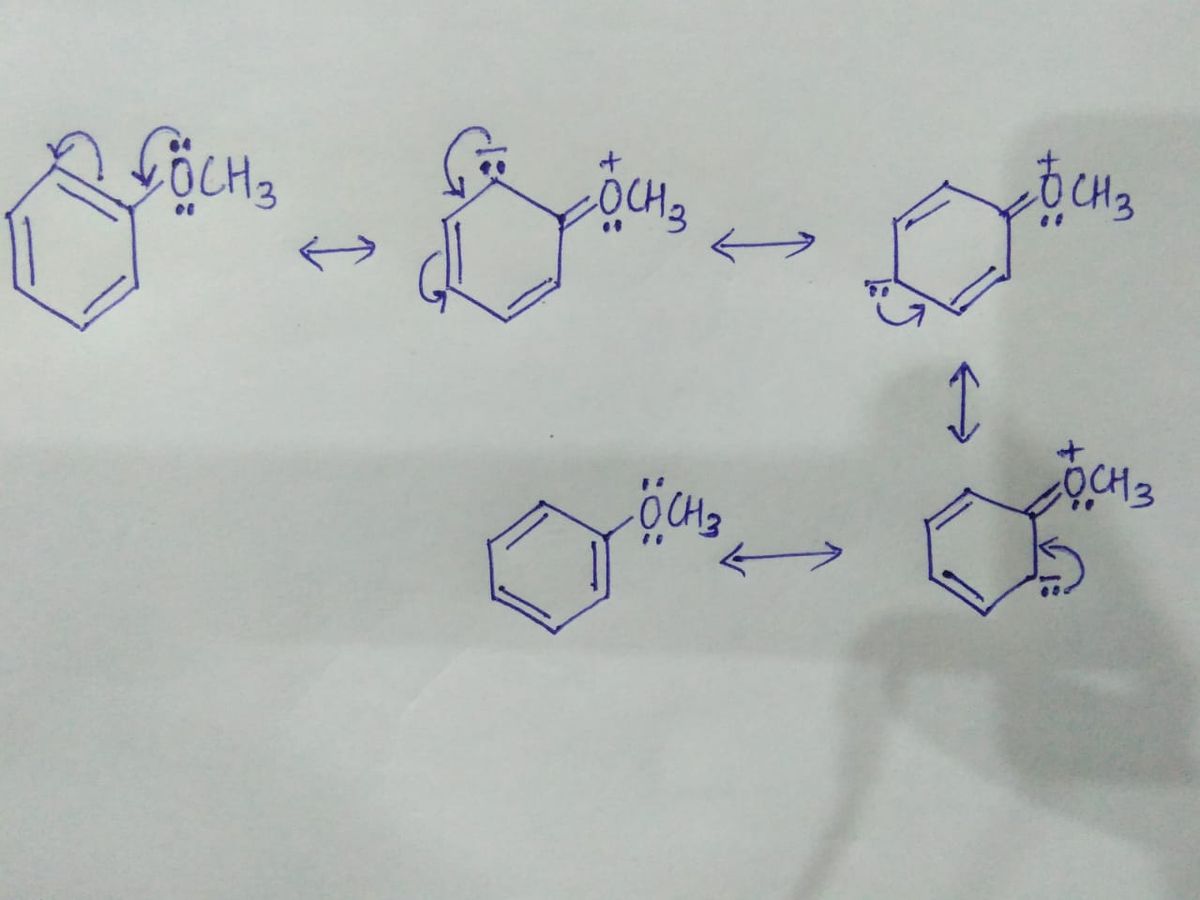
In this compound, the Oxygen atom (which has a lone pair) is directly attached to the benzene ring. Due to which the lone pairs present on the oxygen atom delocalizes in the ring and make ring electron rich. Hence, the given compound show Electron donating resonance effect.
Step by step
Solved in 2 steps with 2 images









