Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

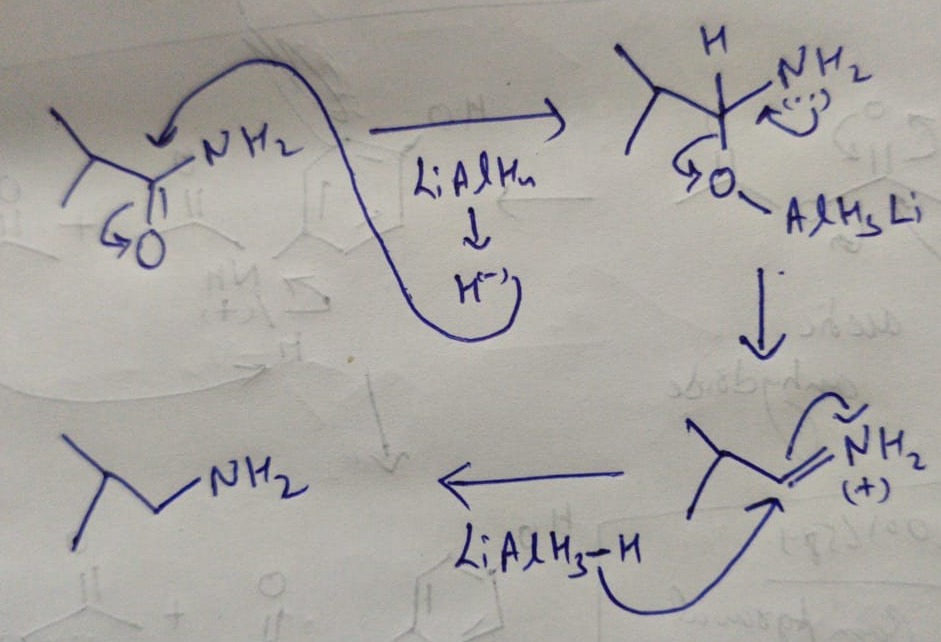
Transcribed Image Text:### Chemical Reaction Illustration
**Reactant:**
- The reactant is an organic molecule with the following structural formula:
- It features an amide functional group (RCONH2), where there is a carbonyl group (C=O) attached to a nitrogen atom (NH2).
- The structure can be represented as follows:
```
O
||
C
/ \
H2N CH3
|
CH3
```
**Reagent:**
- The reagent employed in this reaction is Lithium Aluminium Hydride (LiAlH4).
**Reaction Arrow:**
- A reaction arrow points from the reactant (left side) toward an empty box (right side) where the product of the reaction will appear.
**Explanation:**
- Lithium Aluminium Hydride (LiAlH4) is typically used as a reducing agent. In this case, it is expected to reduce the amide group in the reactant molecule to generate the final product.
- The exact product of the reaction is not shown in the image but based on typical reactions involving LiAlH4 and amides, the likely product would be an amine, where the carbonyl group is replaced by two hydrogen atoms.
### Detailed Description for Educational Context:
- **Amide Reduction with Lithium Aluminium Hydride (LiAlH4):**
- Amides can be reduced to amines using Lithium Aluminium Hydride, a strong reducing agent.
- In this specific reaction, the amide group (R-C=O-NH2) is reduced to an amine (R-CH2-NH2).
- Lithium Aluminium Hydride provides hydrogen atoms that add to the carbonyl carbon and the nitrogen atom, effectively converting the C=O bond into two C-H bonds.
**Key Points to Highlight:**
- Understand the structural components of amides and the role of strong reducing agents like LiAlH4.
- Familiarize with the transformation of functional groups such as the reduction of an amide to an amine.
- The product of this reaction would be an amine, represented structurally as:
```
H2N
|
CH3
|
CH3
```
This educational material aims to provide insights into organic reactions, specifically focusing on the reduction of amides to amines, which is a commonly encountered reaction in organic chemistry.
Expert Solution
Step 1
LiAlH4 is a strong reducing agent as it produces H- ions which can attack on the carbon making bonds with O and N as it as partial +ve charge due to bonding with electronegative atoms O and N.
Step 2
Hence the below reaction with product.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY