Initial Final K, +马+馬,WK y.AE te= 0.80 s Y3D0.70m to Earth ti= 0 Earth mbarbell=13.6 kg వా
Initial Final K, +马+馬,WK y.AE te= 0.80 s Y3D0.70m to Earth ti= 0 Earth mbarbell=13.6 kg వా
Related questions
Question

Transcribed Image Text:EV E Math
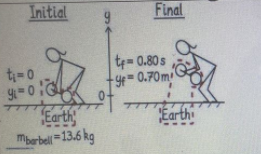
Initial
Final
K, + U, + U, - W- K, - Ug+ Uy
Usr + AEtherm
+.
t= 0.80 s
ti3=D0
y=
0.70m
0-
7777
to
Earth
0.
Earth
Mbarbell = 13.6 kg
8. The weightlifter, not in the system, raises a barbell.
Energy Conservation Equation
Show al
Civitwar politics.. docx
a
At Home Friction.pptx
IMG_1490jpg
2:43 PM
12/9/2020
T||||||||
|||||||
2|||||1|1
||L||||||
1||||||||
1||||||||
||||||
Expert Solution
Step 1
Given:
The mass of the barbell is 13.6 kg.
The diagram is as follows:
Introduction:
The principle of work and kinetic energy states that the work done by the sum of all forces acting on a particle equals the change in the kinetic energy of the particle. This definition can be extended to rigid bodies by defining the work of the torque and rotational kinetic energy.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images
