in 225. mL of a solvent with a density of 0.96 g/mL. The student notices that the volume of the solvent does not A student dissolves 10. g of styrene (CH₂) change when the styrene dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits. x
in 225. mL of a solvent with a density of 0.96 g/mL. The student notices that the volume of the solvent does not A student dissolves 10. g of styrene (CH₂) change when the styrene dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits. x
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:ome File Edit View History Bookmarks Profiles
Chai
=
G numb G aceto
Cell t b Answb Answ My DA AL x CA stur CA sol
C www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNslkr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInMX9hNGVYTIJ JZmF5TTEgJckvK3y58Hg
Solubility and... 18.3 Gibbs Free E... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... SOLUTION: The le... Math 115 W-S Fall...
(51)
OSTATES OF MATTER
Calculating molality
Micro To do Neuro
& list of
□
esc
molarity =
molality = 0
Explanation
1
Q
A
2
A student dissolves 10. g of styrene (CH₂) in 225. mL of a solvent with a density of 0.96 g/mL. The student notices that the volume of the solvent does not
change when the styrene dissolves in it.
Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.
Z
Check
W
Tab
S
8
#
3
X
Window
E
0° 0x0
17
D
$
4
C
5
R
F
%
5
V
tv
A
6
T | Y
G
B
&
7
H
CE
U
N
8
—
J
M
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
97 A
9
▬▬
O
KL
<
1
0
H
Get started.: Hyp
P
0 ☆
command
0/5
Aa
G 96/1
alt
..
option
2020
La
(
80
1
Expert Solution
Step 1
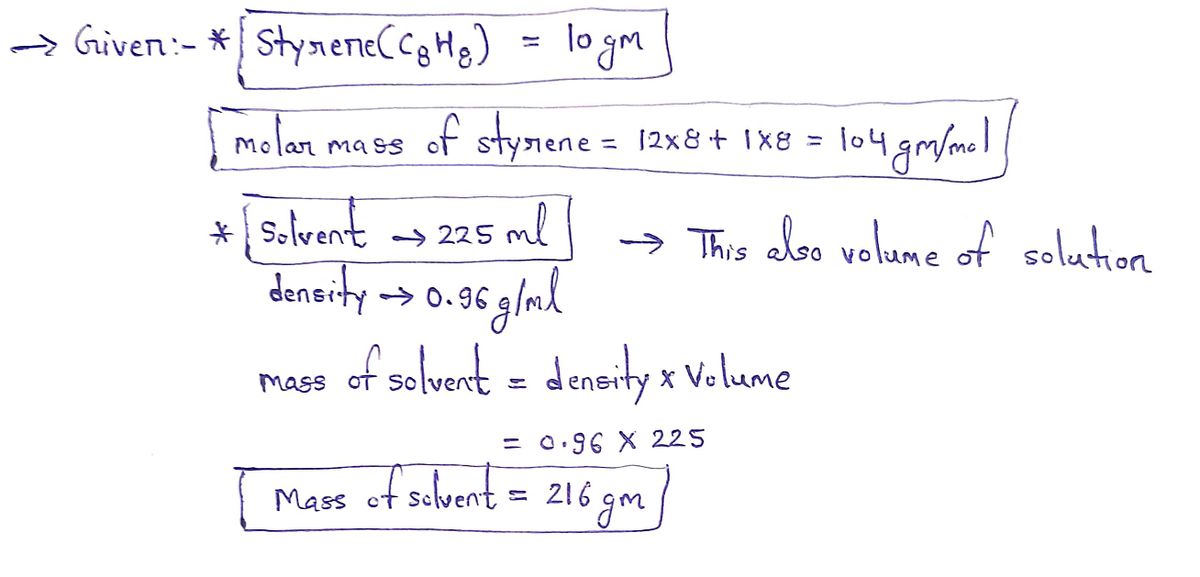
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY