Imagine that the generic reaction shown below has a ΔG′° = 0. Will this reaction proceed spontaneously in the direction written under the conditions given below? A + B → C + D [A] = 0.1 M[B] = 0.8 M[C] = 0.2 M[D] = 0.3 M No, the reaction is at equilibrium More information is needed to answer this question No, it will proceed spontaneously in the reverse direction Yes, it will proceed spontaneously in the direction written It depends on the temperature
Imagine that the generic reaction shown below has a ΔG′° = 0. Will this reaction proceed spontaneously in the direction written under the conditions given below?
A + B → C + D
[A] = 0.1 M
[B] = 0.8 M
[C] = 0.2 M
[D] = 0.3 M
No, the reaction is at equilibrium
More information is needed to answer this question
No, it will proceed spontaneously in the reverse direction
Yes, it will proceed spontaneously in the direction written
It depends on the temperature
A reaction is said to be in equilibrium if the rate of forward reaction is equal to the rate of the backward reaction. The reaction quotient determines the direction in which the system will proceed to reach equilibrium. It is represented by an expression as shown below:
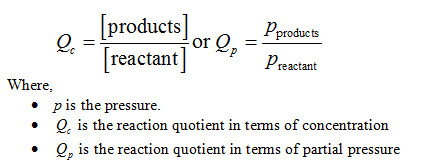
Given data:
[A] = 0.1 M
[B] = 0.8 M
[C] = 0.2 M
[D] = 0.3 M
The reaction is given as follows:
A + B → C + D
The reaction quotient for the reaction is calculated as follows:
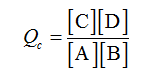
Step by step
Solved in 3 steps with 3 images









