Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
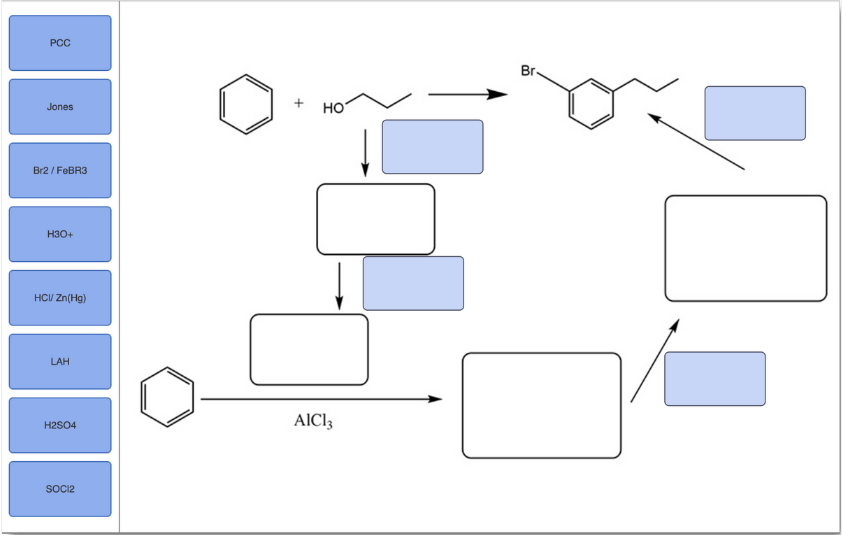
Identify the reagents needed to complete this multistep synthesis.

Transcribed Image Text:The image displays a flowchart of a chemical synthesis process, featuring benzene and an alcohol derivative reacting to form a brominated compound. The process involves several reactions that are to be completed using specified reagents.
### Reagents:
- **PCC (Pyridinium chlorochromate)**
- **Jones Reagent**
- **Br₂/FeBr₃ (Bromine and Iron(III) bromide)**
- **H₃O⁺ (Hydronium ion)**
- **HCl/Zn(Hg) (Hydrochloric acid and zinc amalgam)**
- **LAH (Lithium aluminium hydride)**
- **H₂SO₄ (Sulfuric acid)**
- **SOCl₂ (Thionyl chloride)**
### Chemical Structures and Arrows:
1. **Benzene** is shown reacting with an alcohol derivative.
2. **Br₂/FeBr₃** reagent is indicated to be responsible for transforming the compound into a brominated benzene ring.
3. An intermediate with a hydroxy group suggests a possible nucleophilic substitution or elimination step.
4. **AlCl₃ (Aluminum chloride)** is used further along in the pathway, possibly indicating a Friedel-Crafts alkylation or acylation.
5. Several other reaction steps with empty placeholders indicate directions where different reagents from the list might be applied.
The intention is to fill in these reaction steps with appropriate reagents and intermediates based on knowledge of organic chemistry reactions and mechanisms.
The flowchart should be explored to understand how reagents interact to carry out specific transformation steps in synthesis.
Expert Solution
Step 1
Given that :
We have to identify the reagents needed to complete the following multistep synthesis :
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY