I Review I Constants I Periodic Tab MISSED THIS? Read Section 16.8 (Pages 701 -710) ; Watch KCV 16.8, IWE 16.9 . For the reaction Part A N20.(g)= 2NO,(g) Ha reaction vessel initially contains an N20, concentration of 0.0500 M at 500 K, what are the equilibrium concentrations of N30, and NO, at 500 K? Ke=0.513 at 500 K. Express your answers in moles per liter to three significant figures separated by a comma. > View Avalable Hint(s) N,O]- [NO;] = M Submit
I Review I Constants I Periodic Tab MISSED THIS? Read Section 16.8 (Pages 701 -710) ; Watch KCV 16.8, IWE 16.9 . For the reaction Part A N20.(g)= 2NO,(g) Ha reaction vessel initially contains an N20, concentration of 0.0500 M at 500 K, what are the equilibrium concentrations of N30, and NO, at 500 K? Ke=0.513 at 500 K. Express your answers in moles per liter to three significant figures separated by a comma. > View Avalable Hint(s) N,O]- [NO;] = M Submit
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![Review I Constants I Periodic Table
MISSED THIS? Read Section 16.8 (Pages 701 - 710) : Watch
KCV 16.8, IWE 16.9.
For the reaction
Part A
N204(g) = 2NO2(g)
Ha reaction vessel initially contains an N,O4 concentration of 0.0500 M at 500 K, what are the equilibrium concentrations of N3O4 and NO, at 500 K?
K. = 0.513 at 500 K.
Express your answers in moles per liter to three significant figures separated by a comma.
> View Available Hint(s)
?
N,OJ, [NO;] =
M
Submit
< Retum to Assignment
Provide Feedback
MacBook Air
esc
F1
&
#3
%24
3
4.
5
6.
8.
W
E
R](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5ff25e28-5246-4e63-a972-d5fbafce3c9e%2F440cfa9f-2f7d-43cb-b848-28f4b0fbf43b%2Fzubxsq_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Review I Constants I Periodic Table
MISSED THIS? Read Section 16.8 (Pages 701 - 710) : Watch
KCV 16.8, IWE 16.9.
For the reaction
Part A
N204(g) = 2NO2(g)
Ha reaction vessel initially contains an N,O4 concentration of 0.0500 M at 500 K, what are the equilibrium concentrations of N3O4 and NO, at 500 K?
K. = 0.513 at 500 K.
Express your answers in moles per liter to three significant figures separated by a comma.
> View Available Hint(s)
?
N,OJ, [NO;] =
M
Submit
< Retum to Assignment
Provide Feedback
MacBook Air
esc
F1
&
#3
%24
3
4.
5
6.
8.
W
E
R
Expert Solution
Step 1
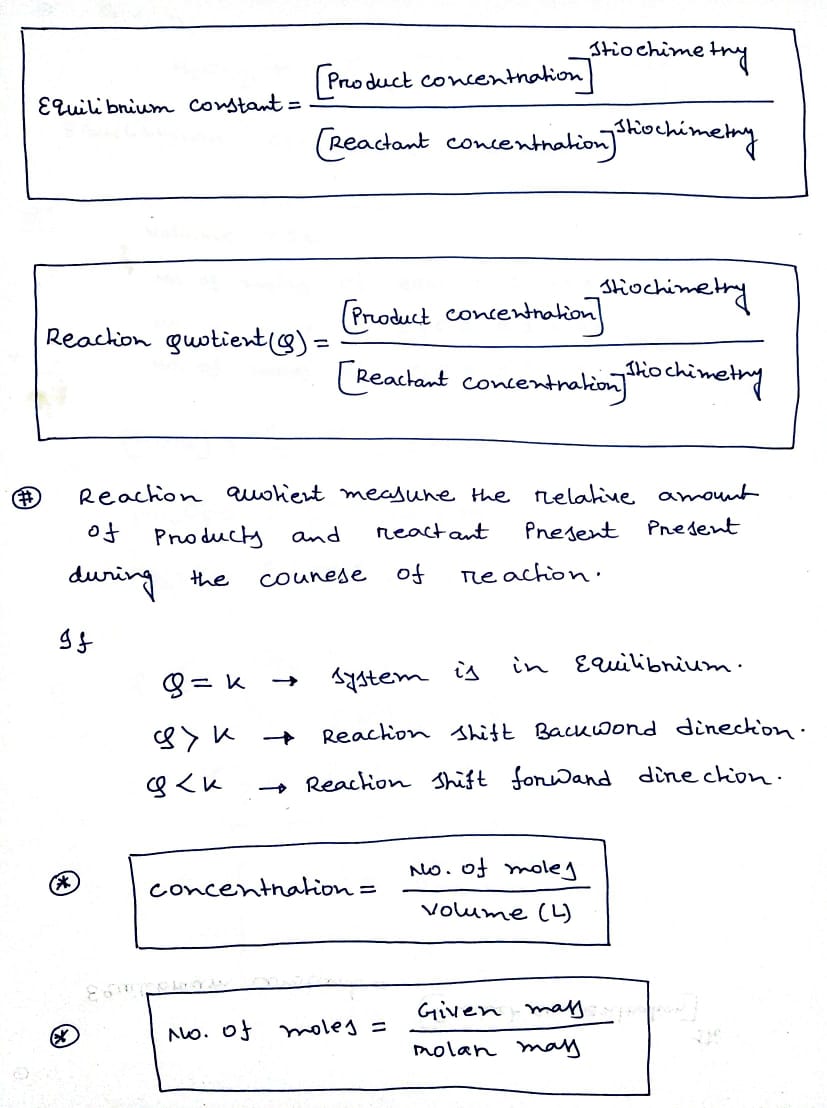
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY