I need to find volumectric flow rate. I attached my work as well as the question. I feel like my process is correct, but maybe I got lost in a math step.
I need to find volumectric flow rate. I attached my work as well as the question. I feel like my process is correct, but maybe I got lost in a math step.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
I need to find volumectric flow rate. I attached my work as well as the question. I feel like my process is correct, but maybe I got lost in a math step.
![The image contains a set of thermodynamic equations, most likely related to energy balance and mass flow in a control volume. Here is the transcription and explanation:
### Mathematical Equations
1. **Energy Balance Equation:**
\[
\sum \dot{m_i} (h_i + g z_i + \frac{y_i^2}{2}) + \sum \dot{m_o} (h_o + g z_o + \frac{y_o^2}{2}) - \sum \dot{m_f} (h_f + g z_f + \frac{y_f^2}{2}) + \dot{Q} + \dot{W} = \frac{dU}{dt}
\]
2. **Mass Flow Equation:**
\[
\dot{m}_1 h_1 + \dot{m}_2 h_2 - \dot{m}_2 h_2 - \dot{m}_4 h_4 = 0
\]
3. **Specific Enthalpy Relations:**
\[
\dot{m}_2 (h_1 - h_2) + \dot{m}_3 (h_3 - h_4) = 0
\]
4. **Energy Transfer Equation:**
\[
\dot{m}_2 (h_1 - h_2) = \dot{m}_{34} (h_4 - h_3)
\]
5. **Heat Transfer Equation:**
\[
\dot{n} C_p (T_1 - T_2) = \dot{m}_{34} (h_w - h_g)
\]
- Rearranged:
\[
\dot{n} C_p (T_1 - T_2) \over (h_w - h_g) = \dot{m}_{34}
\]
6. **Mass Flow of Fluid:**
\[
\dot{m}_3 = 0.95 \, \text{kg/s} \cdot \frac{\cos(107.3^\circ)}{1 \, \text{kg}} \cdot \frac{k_{used}}{2m^2} = 0.101
\]
### Additional Notes
- **Symbols Used:**
- \(\dot{m}\):](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc77f544a-ee3e-4902-8958-66e53c68e4a2%2Fbb057f7d-2c8a-4adf-97cf-ebe880bc497b%2Ft4emagk_processed.png&w=3840&q=75)
Transcribed Image Text:The image contains a set of thermodynamic equations, most likely related to energy balance and mass flow in a control volume. Here is the transcription and explanation:
### Mathematical Equations
1. **Energy Balance Equation:**
\[
\sum \dot{m_i} (h_i + g z_i + \frac{y_i^2}{2}) + \sum \dot{m_o} (h_o + g z_o + \frac{y_o^2}{2}) - \sum \dot{m_f} (h_f + g z_f + \frac{y_f^2}{2}) + \dot{Q} + \dot{W} = \frac{dU}{dt}
\]
2. **Mass Flow Equation:**
\[
\dot{m}_1 h_1 + \dot{m}_2 h_2 - \dot{m}_2 h_2 - \dot{m}_4 h_4 = 0
\]
3. **Specific Enthalpy Relations:**
\[
\dot{m}_2 (h_1 - h_2) + \dot{m}_3 (h_3 - h_4) = 0
\]
4. **Energy Transfer Equation:**
\[
\dot{m}_2 (h_1 - h_2) = \dot{m}_{34} (h_4 - h_3)
\]
5. **Heat Transfer Equation:**
\[
\dot{n} C_p (T_1 - T_2) = \dot{m}_{34} (h_w - h_g)
\]
- Rearranged:
\[
\dot{n} C_p (T_1 - T_2) \over (h_w - h_g) = \dot{m}_{34}
\]
6. **Mass Flow of Fluid:**
\[
\dot{m}_3 = 0.95 \, \text{kg/s} \cdot \frac{\cos(107.3^\circ)}{1 \, \text{kg}} \cdot \frac{k_{used}}{2m^2} = 0.101
\]
### Additional Notes
- **Symbols Used:**
- \(\dot{m}\):

Transcribed Image Text:### Heat Exchanger Problem
**Problem Statement:**
Steam is used to pre-heat an air stream before it is fed into a combustion chamber. Steam enters the well-insulated heat exchanger at 415 °C and 300.0 kPa and is isobarically condensed to a saturated liquid. The air enters at 45.0 °C and 125.0 kPa with a rate of 1050 L/s and must be heated to 255.0 °C. Assume air is an ideal gas with MW = 29 and \( C_p = 3.5 \times R \).
**Questions:**
1. **What is the flowrate of water leaving the exchanger?**
- **Submitted Answer:** -0.102 L/s
- **Status:** Incorrect
2. **What is the heat interaction term for the water?**
- **Submitted Answer:** -260.98 kJ/s
- **Status:** Incorrect
**Feedback:**
- Ensure you use the correct specific volume for the exiting water stream.
- Double-check the expected sign on the water stream. Should it be negative or positive?
Expert Solution
Heat transfer digram and calculation of LMTD
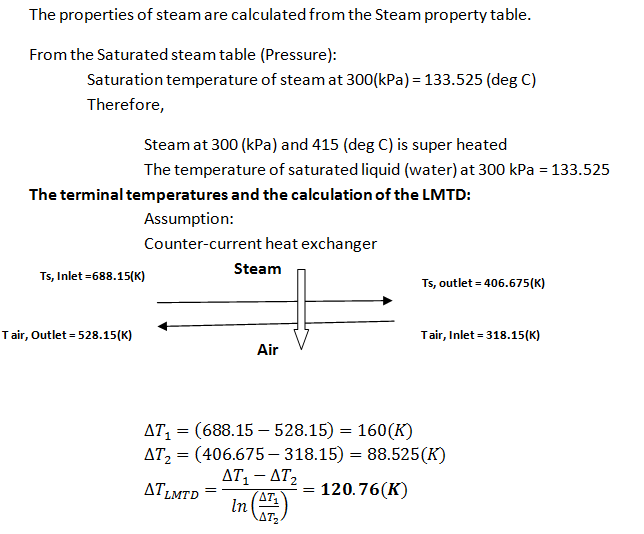
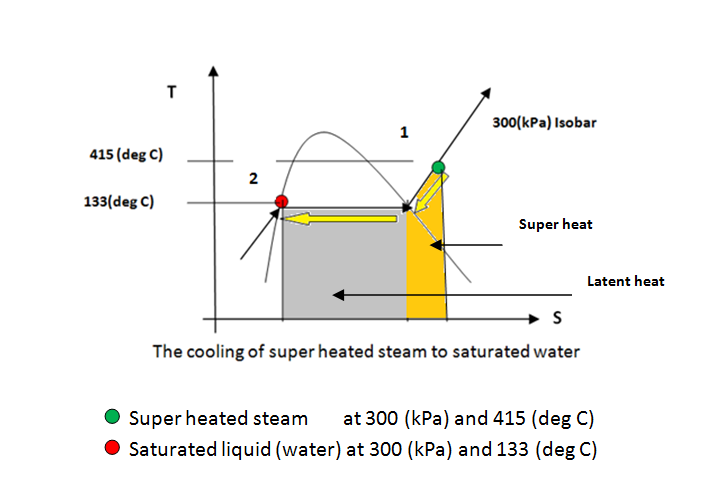
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 7 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The