Carbohydrates
Carbohydrates are the organic compounds that are obtained in foods and living matters in the shape of sugars, cellulose, and starch. The general formula of carbohydrates is Cn(H2O)2. The ratio of H and O present in carbohydrates is identical to water.
Starch
Starch is a polysaccharide carbohydrate that belongs to the category of polysaccharide carbohydrates.
Mutarotation
The rotation of a particular structure of the chiral compound because of the epimerization is called mutarotation. It is the repercussion of the ring chain tautomerism. In terms of glucose, this can be defined as the modification in the equilibrium of the α- and β- glucose anomers upon its dissolution in the solvent water. This process is usually seen in the chemistry of carbohydrates.
L Sugar
A chemical compound that is represented with a molecular formula C6H12O6 is called L-(-) sugar. At the carbon’s 5th position, the hydroxyl group is placed to the compound’s left and therefore the sugar is represented as L(-)-sugar. It is capable of rotating the polarized light’s plane in the direction anticlockwise. L isomers are one of the 2 isomers formed by the configurational stereochemistry of the carbohydrates.
How to Assign R,S designations to stereogenic center in glucose ?
Glucose is a monosaccharide, having formula C6H1206. It is an important simple sugar that circulates in the blood of living beings as blood sugar Glucose, having 6 carbon atoms and an aldehyde group, is an aldo-hexose.
The structure of D-glucose can be written as
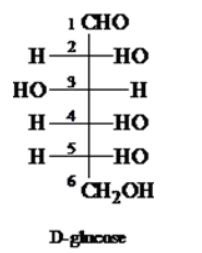
To assign give R, S designations to each stereogenic centre in glucose, follow the given steps
To find the R or S configuration of stereocenters present in D-glucose, priority order needs to be assigned to the compound by using the following CIP (Cahn-Ingold-Prelog) sequence rules
Chirality center: An atom (usually carbon) which is bonded to four different atoms or groups is known as chiral center.
Rule 1: Select the chiral center in the compound and assign priorities to the atoms based on their atomic number. The atom which has highest atomic number gets the first priority and atom which is having least atomic number (usually hydrogen) gets the fourth priority.
Rule 2: If the first atom of each substituent is same then the next atom is considered for assigning the priority This process is continued to the third and fourth atom until the point of difference is reached
Rule 3: If the substituents have multiple bonds, the multiple bonded atoms are considered as same number of single bonded atoms
If the priority order of the substituents from highest to second highest to third highest 1-->2--> 3 is in clock wise direction then the chiral center has R configuration (Latin rectus means right).
If the priority order of the substituents from highest to second highest to third highest 1--> 2--> 3 is counter clock wise direction then the chiral center has S configuration (Latin sinister means left).
If the least priority substituent (usually hydrogen) is present on the horizontal line then its configuration is reversed (that is clockwise becomes S and anticlockwise R).
Step by step
Solved in 2 steps with 2 images









