How temperature affects density? Please explain
Density always holds inverse relation with volume and the volume holds direct connection with temperature. The higher density reflects how dense any object is or how close the particles exist in any object.
The formula or the density is shown below.
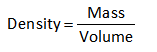
It is clear from the above expression that the density carries direct relation with volume. An increment in substance’s volume will cause the decrement in its density and vice-versa.
When any substance gets heated up its weight (mass) will be same (constant) but the volume basically gets changed. On rising its temperature the space held by substance’s particles will increase and thus the expansion in that its volume will occur. At this state that substance will contain larger volume and thus its density will become less.
For example- hot air carries less density whereas cold air carries high density because the hot air’s volume is higher due to its high temperature, the particles exist at far distance so that hot air contains low density. And cold air’s volume is lesser due to its low temperature, the particles exist at close distance thus the cold air contains high density.
Step by step
Solved in 3 steps with 1 images









