Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
How much Mol S2O32- consumed and how much I3- was produced?

Transcribed Image Text:Data Tables for Kinetics Lab
There are a BUNCH of calculations for this lab. I've organized the data into the table below. You will need to read the table in the.
procedure VERY carefully to make sure you're using the correct volumes and concentrations for each calculation below.
Many of the points for this lab will come from the submission of this data sheet with the calculations and the two graphs! If your
data is incorrect on these tables, your graphs will be incorrect and you will not get the correct values for the order of the reaction!
Make sure you know which sulfur compound is which!
Thiosulfate (from the procedure) is S₂0,²
Persulfate (from the procedure) is also called peroxosulfate or peroxodisulfate and is $₂O²
Data table 1 A
Trial Total
1
2
3
4
5
volume
upon
mixing
flask A +
flask B
15m
65mL
SML
Mol S₂0₁2
consumed
(all S₂0₂²
added is
consumed)
Molly
produced
(according
to the
background
this is the
mol S₂03²-
consumed)
Show your work for the calculations for Trial 1.
Color right Time to
after
mixing
color
change
clear
Clear
Char
0.2 mx 25ml
لہ کیا
Final Color
35see black blee
50sec black/blen
100 sec black blue
sosee lack the
100sec black/blue
= 0.0769m
Trial 1 25
Trial 2 25
Flask A
Volume of Volume of Volume Volume of
0.20 M 0.20 M
of 0.20 M
ammonium
potassium potassium 0.2%
iodide (mL) nitrate (mL) starch persulfate
(mL) (mL)
Trial 3 25
Trial 4
Trial 5
12.5
6.25
0
0
0
12.5
Flask Contents
18.75
5
5
5
5
5
25
12.5
6.25
25
25
Flask B
Volume of Volume of
0.20 M 0.010 M
ammonium sodium
sulfate (mL) thiosulfate
(mL)
0
12.5
18.75
0
0
10
10
10
10
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
I also need help filling out these.
![Use the Mol S2032 consumed and Mol 13 produced from the table above for the calculations in Table 2
Since you are working with moles from Table 1, you'll need to divide by total volume in liters to get the [S₂032] and [13].
Since you are working only with M and V for the persulfate [S2082] and iodide [1] and just diluting solutions, you can use mL
in your M₂V₁-M₂V₂ calculations. Or you can use L, just use the same unit for both!
●
Data table 2 B
Trial
1
2
3
4
5
thiosulfate [13]
[S203²]
consumed
(remember
to divide the
moles S₂03²-
by the TOTAL
volume, not
the vol you
added)
produced
(remember
to divide the
moles 13 by
the TOTAL
volume, not
the vol you
added)
Persulfate
[S₂08²-] at
mixing
(use
M₁V1=M₂V2
with total
volume)
Show your work for the calculations for Trial 1:
[1] at
mixing
(use
M₁V₁=M₂
V2 with
Log[S₂082] Log[1]
Rate=
Δ[13]
At
total replaced by numbers in the Postb assig
volume)
You will need to plot two different graphs in Excel and attach them in the Postlab:
Log(rate)
on these in](https://content.bartleby.com/qna-images/question/364f8f4a-7362-494d-a7c0-26feeadafcdd/2c4d3933-c8a8-483a-9d21-f91b300f2230/oqup2j_thumbnail.jpeg)
Transcribed Image Text:Use the Mol S2032 consumed and Mol 13 produced from the table above for the calculations in Table 2
Since you are working with moles from Table 1, you'll need to divide by total volume in liters to get the [S₂032] and [13].
Since you are working only with M and V for the persulfate [S2082] and iodide [1] and just diluting solutions, you can use mL
in your M₂V₁-M₂V₂ calculations. Or you can use L, just use the same unit for both!
●
Data table 2 B
Trial
1
2
3
4
5
thiosulfate [13]
[S203²]
consumed
(remember
to divide the
moles S₂03²-
by the TOTAL
volume, not
the vol you
added)
produced
(remember
to divide the
moles 13 by
the TOTAL
volume, not
the vol you
added)
Persulfate
[S₂08²-] at
mixing
(use
M₁V1=M₂V2
with total
volume)
Show your work for the calculations for Trial 1:
[1] at
mixing
(use
M₁V₁=M₂
V2 with
Log[S₂082] Log[1]
Rate=
Δ[13]
At
total replaced by numbers in the Postb assig
volume)
You will need to plot two different graphs in Excel and attach them in the Postlab:
Log(rate)
on these in
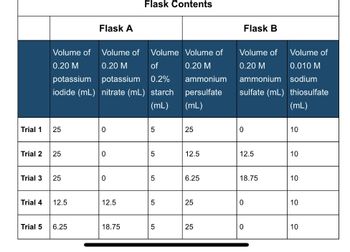
Transcribed Image Text:Trial 1
Trial 2
Trial 3
Trial 4
Trial 5
25
Volume of
Volume of
Volume
0.20 M
0.20 M
of
potassium
potassium
0.2%
iodide (mL) nitrate (mL) starch
(mL)
25
25
12.5
Flask A
6.25
0
0
0
12.5
Flask Contents
18.75
5
5
5
5
01
5
Volume of
0.20 M
ammonium
persulfate
(mL)
25
12.5
6.25
25
25
Flask B
Volume of
0.20 M
ammonium
sulfate (mL)
0
12.5
18.75
0
0
Volume of
0.010 M
sodium
thiosulfate
(mL)
10
10
10
10
10
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY