How many g of Si3O5 are in 0.094 mol Si3O5?
SHOW WORK. Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for this problem. Abbreviate units as follows: g, mol, molec, atom.
If molar mass is needed, be sure to use our periodic table and round to the proper number of decimal places.
If
Use the three blanks to enter the number, unit, and substance (in this order) that appears in the numerator of the conversion factor.
Calculate the answer and use the first two blanks to report the value (scientific notation including proper s.f., and exponent), and the final two blanks to report units and substance (in this order).
× 10
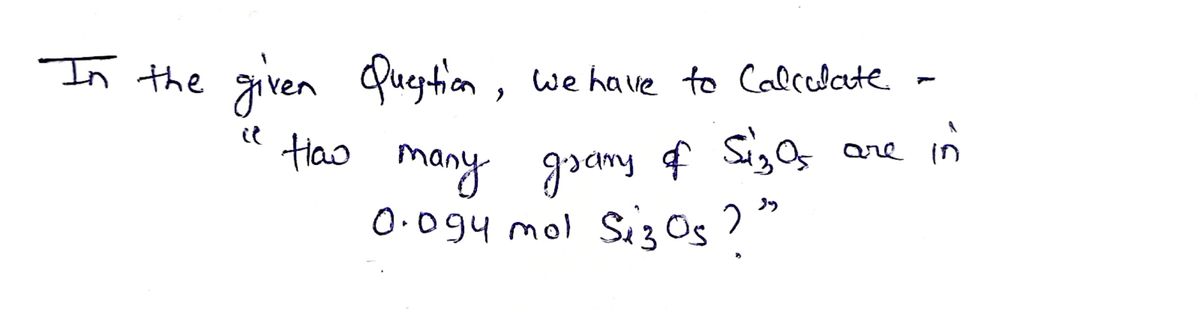
Step by step
Solved in 2 steps with 2 images









