Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
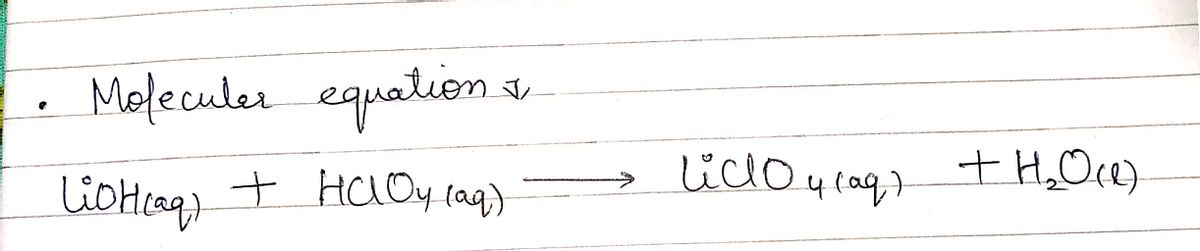
![**Exercise: Writing a Net Ionic Equation**
**Objective:**
Write the net ionic equation for the neutralization of lithium hydroxide (LiOH) by aqueous perchloric acid (HClO₄).
**Instructions:**
- Use the lowest possible coefficients.
- Specify the states of each compound, such as (aq) for aqueous or (s) for solid.
- If a box is not needed, leave it blank.
- Use H⁺ for the hydronium ion.
**Equation Template:**
\[ \boxed{} + \boxed{} \rightarrow \boxed{} + \boxed{} \]
**Steps:**
1. Identify the ions present in the reactants and products.
2. Cancel out the spectator ions to find the net ionic equation.
3. Fill in the provided boxes with the appropriate ions or compounds, including their states.
**Submission:**
- Click "Submit" when ready.
- You have 3 attempts remaining for this submission.
**Controls:**
- [Submit Answer]
- [Try Another Version]
- Navigate using [Previous] and [Next] buttons.
**Additional Instructions:**
- Save your progress using the "Save and Exit" option.
- Access supplementary resources as needed.
Happy learning!](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F924f1927-2b95-447c-8315-cf80b16c3d9e%2F854aefc1-fb0d-4f69-9634-13446a730a5f%2Fehrn6p_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Exercise: Writing a Net Ionic Equation**
**Objective:**
Write the net ionic equation for the neutralization of lithium hydroxide (LiOH) by aqueous perchloric acid (HClO₄).
**Instructions:**
- Use the lowest possible coefficients.
- Specify the states of each compound, such as (aq) for aqueous or (s) for solid.
- If a box is not needed, leave it blank.
- Use H⁺ for the hydronium ion.
**Equation Template:**
\[ \boxed{} + \boxed{} \rightarrow \boxed{} + \boxed{} \]
**Steps:**
1. Identify the ions present in the reactants and products.
2. Cancel out the spectator ions to find the net ionic equation.
3. Fill in the provided boxes with the appropriate ions or compounds, including their states.
**Submission:**
- Click "Submit" when ready.
- You have 3 attempts remaining for this submission.
**Controls:**
- [Submit Answer]
- [Try Another Version]
- Navigate using [Previous] and [Next] buttons.
**Additional Instructions:**
- Save your progress using the "Save and Exit" option.
- Access supplementary resources as needed.
Happy learning!
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY