Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
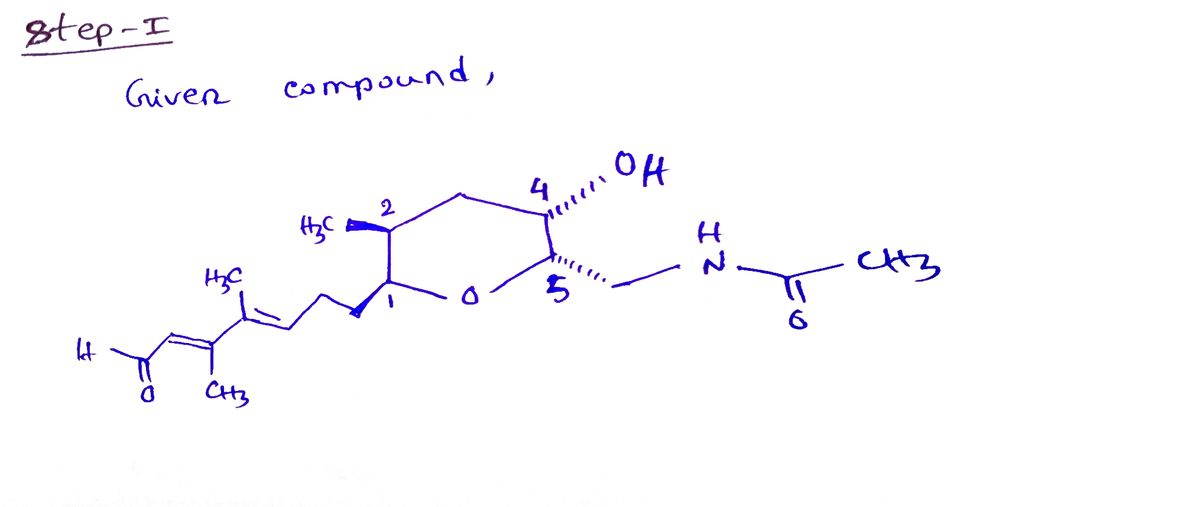
List the absolute stereochemistry at carbons 1,2,4 and five

Transcribed Image Text:The image shows the chemical structure of brevisamide. Below is an explanation of the structure:
**Chemical Structure Details:**
1. **Molecular Framework:**
- Brevisamide has a cyclic backbone and contains a six-membered ring.
- The structure prominently features both double and single bonds.
2. **Functional Groups:**
- There is a hydroxyl group (OH) attached to carbon 4.
- An amide group is present, where the nitrogen is connected to the carbon chain and a carbonyl group (C=O) with a methyl group (CH₃).
3. **Numbering:**
- The carbons in the central ring are numbered 1 through 5 for clarity.
- This helps identify where different groups are attached.
4. **Stereochemistry:**
- The structure includes wedges and dashes indicating stereochemistry at carbons 4 and 5, illustrating the 3D orientation of the bonds.
5. **Side Chain:**
- There is an extended side chain with several methyl groups (CH₃) and a double bond with a terminal carbonyl group featuring an aldehyde hydrogen (H).
This compound can be studied for its potential biochemical or pharmaceutical applications.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY