Carbohydrates
Carbohydrates are the organic compounds that are obtained in foods and living matters in the shape of sugars, cellulose, and starch. The general formula of carbohydrates is Cn(H2O)2. The ratio of H and O present in carbohydrates is identical to water.
Starch
Starch is a polysaccharide carbohydrate that belongs to the category of polysaccharide carbohydrates.
Mutarotation
The rotation of a particular structure of the chiral compound because of the epimerization is called mutarotation. It is the repercussion of the ring chain tautomerism. In terms of glucose, this can be defined as the modification in the equilibrium of the α- and β- glucose anomers upon its dissolution in the solvent water. This process is usually seen in the chemistry of carbohydrates.
L Sugar
A chemical compound that is represented with a molecular formula C6H12O6 is called L-(-) sugar. At the carbon’s 5th position, the hydroxyl group is placed to the compound’s left and therefore the sugar is represented as L(-)-sugar. It is capable of rotating the polarized light’s plane in the direction anticlockwise. L isomers are one of the 2 isomers formed by the configurational stereochemistry of the carbohydrates.
Answer the following question about monosaccharide A.
What product is formed when A undergoes a Wohl degradation?

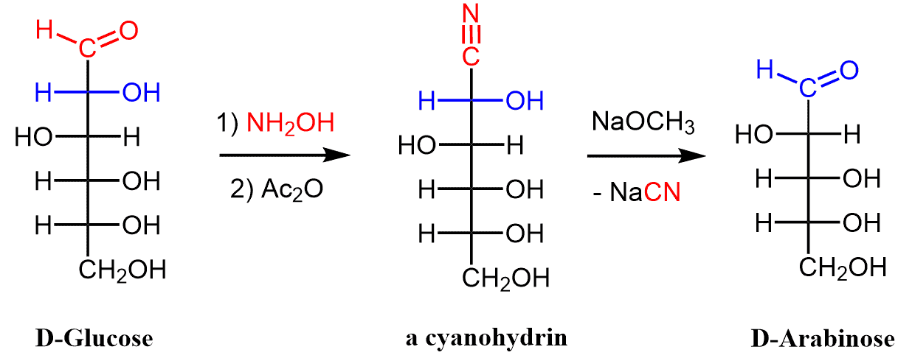
Wohl degradation in carbohydrate chemistry is a chain contract process for aldose. D-glucose is a short type of dextrorotative glucose. It is one of the two glucose stereoisomers, and it is the one that is biologically active. It exists as a result of photosynthesis in plants. It is the product of the degradation of glycogen in animals and fungi. D-Glucose is among the sixteen stereoisomers of aldohexoses. D-isomer, d-glucose, also known as dextrose, appears commonly in nature, but l-isomer, l-glucose, does not exist. Glucose may be obtained through hydrolysis of carbohydrates such as milk sugar (lactose), cane sugar (sucrose), maltose, cellulose, glycogen, etc.
The classic example is the conversion of D-glucose to arabinose as shown below. The reaction was named after the German chemist Alfred Wohl (1863–1939). Wohl degradation binds the C1 – C2 bond of the aldose chain and lessens it by one carbon. As in the Kiliani–Fischer synthesis, the transition is based on the first conversion of the aldehyde group to cyanohydrin. The ability to decrease (degrade) the aldose chain by one carbon was a significant tool in the carbohydrate elucidation structure. This was normally done by the Ruff technique. An fascinating alternative method, known as the well-being depletion, has also been used. The following equation shows the application of this method to aldopentose, arabinose.
Step by step
Solved in 3 steps with 1 images









