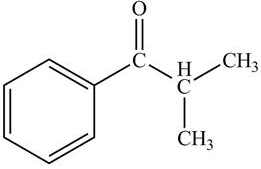
Compound X (molecular formula C10H12O) was treated with NH2NH2, −OH to yield compound Y (molecular formula C10H14). Based on the 1HNMR spectra of X and Y given below, what are the structures of X and Y?

Given:
Molecular formula of compound X=
Molecular formula of compound Y=
The proton NMR peak of compound X which lies near 7 ppm and 8 ppm. It shows that there is the presence of aromatic ring which is substituted by one group and also there is a bond between the carbon of the benzene ring and a carbonyl carbon, because the chemical shift values are greater than normal.
There is a peak near to 4 ppm and 1 ppm. The proton NMR peak near 4 ppm shows that the carbon atom which has one hydrogen atom. Therefore this peak can splits into septet and it depicts that this carbon atom is connected to two carbon atoms which having three hydrogen atoms each.
The proton NMR peak near 1 ppm corresponds to 2 carbon atoms which contains three hydrogen atoms each. This peak splits into doublet, that depicts that this carbon atom is connected to a carbon atom which contains one hydrogen atom.
Therefore the structure of the compound X will be
Step by step
Solved in 3 steps with 2 images









