Consider the attached two-step reaction:
Question: Draw the structure for the transition state in both steps of the
mechanism ?
![H
H
-H
H-Ci:
[1]
[2]
H.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5f293865-2ac2-43aa-95f7-3aa9a9c681c5%2Fb5f6b169-a1d9-4061-a1bf-72026de1dfd7%2Fkz5fqfh.png&w=3840&q=75)
Cyclohex-1-ene is given and it is an alkene. Alkenes consists double bond which are electron rich, so alkene behaves as a nucleophile. And in the first step, it will attack on HCl and abstract H+ which is electron deficient and break single bond between hydrogen and chlorine and provide electron density to chlorine and chloride ion, Cl- is removed. And double bond of cyclohex-1-ene form three membered transition state with H+ ion.
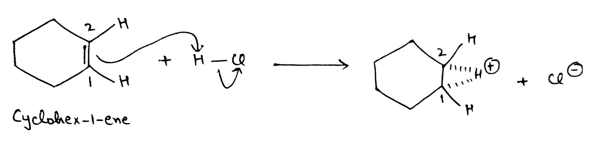
In the second step, three membered transition state break down in such a way that hydrogen is attached to carbon 2 and carbon 1 attains a positive charge.
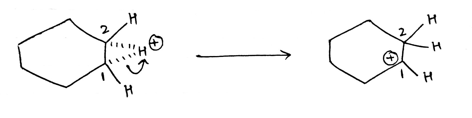
Step by step
Solved in 3 steps with 3 images









