For the given reaction, what volume of O, would required to react with 5.2 L of SO,, measured at the same temperature and pressure? 2 SO,(g) + 0,(g) –→ 2 SO,(g) V =
For the given reaction, what volume of O, would required to react with 5.2 L of SO,, measured at the same temperature and pressure? 2 SO,(g) + 0,(g) –→ 2 SO,(g) V =
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Transcription for Educational Website**
**Problem Statement:**
For the given reaction, what volume of \( O_2 \) would be required to react with 5.2 L of \( SO_2 \), measured at the same temperature and pressure?
**Chemical Reaction:**
\[ 2 \, \text{SO}_2(g) + \text{O}_2(g) \rightarrow 2 \, \text{SO}_3(g) \]
**Required Volume Calculation:**
\[ V = \boxed{\phantom{xxxxxxxx}} \, \text{L} \]
**Explanation:**
For the balanced chemical reaction above, we need to determine the necessary volume of \( O_2 \) gas when reacting with a specified volume of \( SO_2 \) gas. According to the stoichiometry of the reaction, 2 moles of \( SO_2 \) react with 1 mole of \( O_2 \). Using the volume relationship at constant temperature and pressure, the calculation will apply the same molar ratio.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fa563c0cc-a924-4c37-9f6b-c4caff62891a%2Fe29997d0-21d5-4b6c-8561-ae77ace25d37%2Fy98vb9l_processed.png&w=3840&q=75)
Transcribed Image Text:**Transcription for Educational Website**
**Problem Statement:**
For the given reaction, what volume of \( O_2 \) would be required to react with 5.2 L of \( SO_2 \), measured at the same temperature and pressure?
**Chemical Reaction:**
\[ 2 \, \text{SO}_2(g) + \text{O}_2(g) \rightarrow 2 \, \text{SO}_3(g) \]
**Required Volume Calculation:**
\[ V = \boxed{\phantom{xxxxxxxx}} \, \text{L} \]
**Explanation:**
For the balanced chemical reaction above, we need to determine the necessary volume of \( O_2 \) gas when reacting with a specified volume of \( SO_2 \) gas. According to the stoichiometry of the reaction, 2 moles of \( SO_2 \) react with 1 mole of \( O_2 \). Using the volume relationship at constant temperature and pressure, the calculation will apply the same molar ratio.
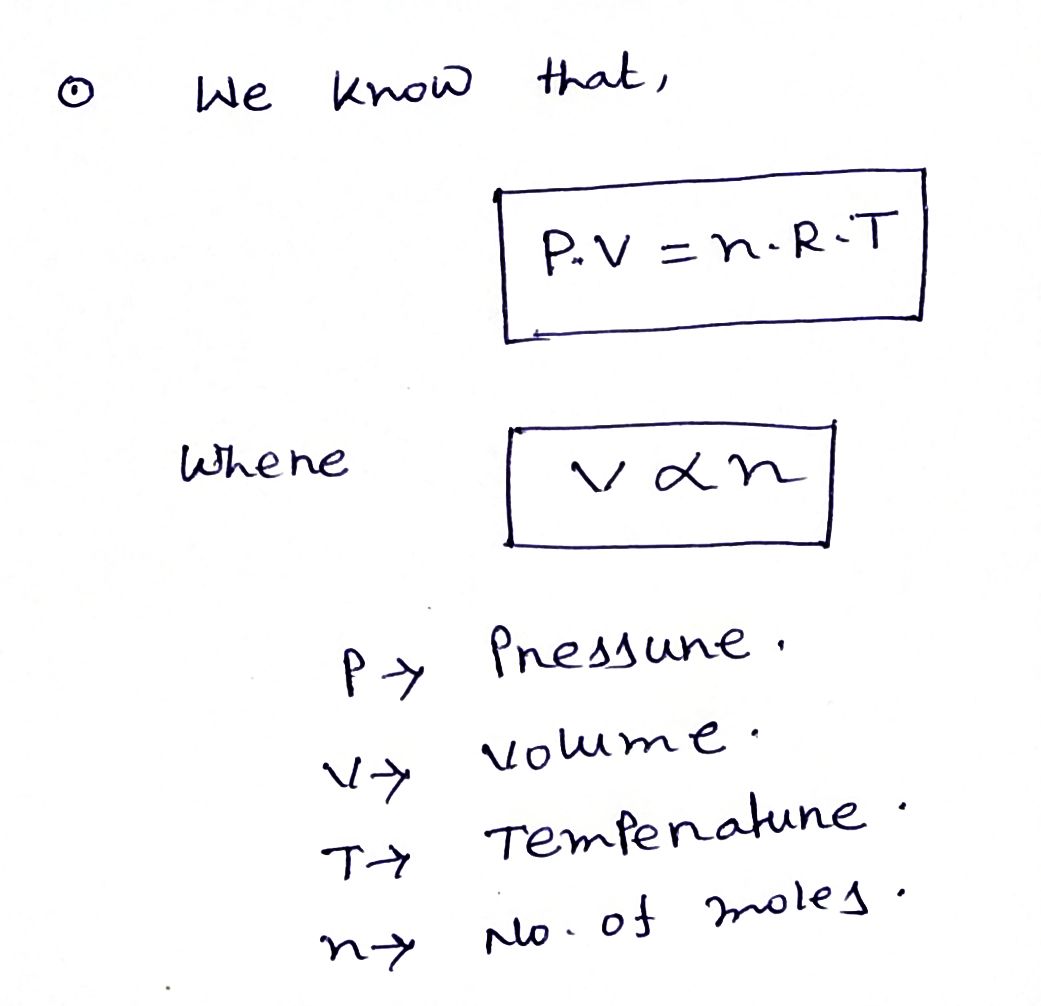
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY