Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
For each of the following reactions give their major product

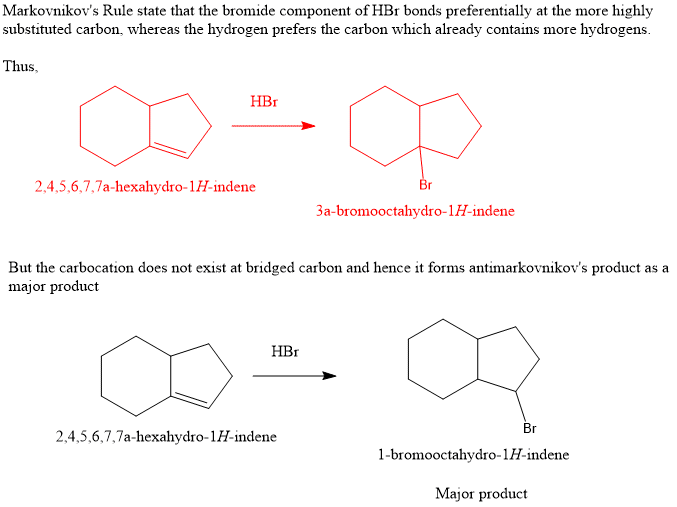
Transcribed Image Text:The image displays a chemical reaction involving a bicyclic alkene, specifically a compound with a six-membered and a five-membered ring fused together, with the smaller ring containing a double bond. The reaction arrow indicates the addition of hydrogen bromide (HBr) across this double bond.
Key Details:
- Reactant: Bicyclic alkene with a six-membered (cyclohexane) and a five-membered (cyclopentene) ring. The double bond is located in the cyclopentene ring.
- Reagent: Hydrogen bromide (HBr), an acid used to add across the double bond in an electrophilic addition reaction.
This type of reaction typically results in the addition of the hydrogen (H) atom to one carbon and the bromine (Br) atom to the other carbon of the double bond, following Markovnikov's rule.

Transcribed Image Text:The image depicts a chemical reaction sequence involving an alkene undergoing a two-step process:
1. **First Step**: The alkene reacts with Mercury(II) acetate \((\text{Hg(OAc)}_2)\) in methanol \((\text{CH}_3\text{OH})\).
2. **Second Step**: The intermediate product from the first step is treated with sodium borodeuteride \((\text{NaBD}_4)\).
This sequence is typically used for oxymercuration-demercuration reactions, where the alkene is converted into an alcohol. The use of \(\text{NaBD}_4\) suggests that the hydrogen atom will be introduced as deuterium (a heavier isotope of hydrogen) in the alcohol product.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY