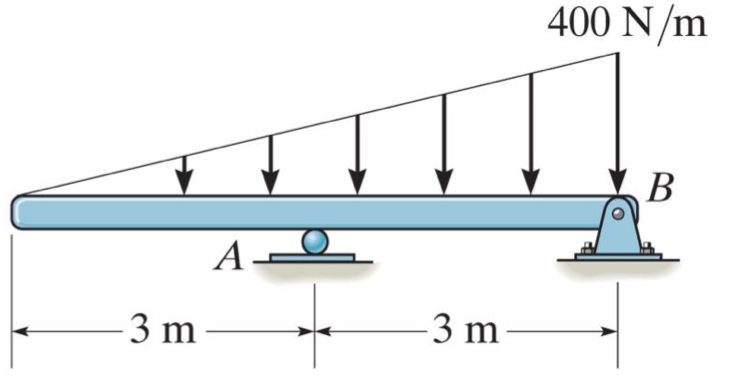
Find the external reactions 400 N/m -3 m AL -3 m- B
Q: How many neutrons does the following isotope of oxygen have? O 18 0 8 O 10 O 26 O 18
A: Given data: The isotope of oxygen O818 Required: Number of neutrons
Q: How many protons & neutrons do the following have? 112 - Cd bromine – 80 48
A: Given 48Cd112here number of protons p = 48number of neutrons n = atomic mass - number of protonsn…
Q: Shielding needed for alpha radiation Shielding needed for beta radiation Shielding needed for gamma…
A: Alpha particle is helium nucleus hence a heavy particle, and it has a very short range. Alpha…
Q: Determine the reactions at A and B for the beams loaded as shown in Figure Beam weight may be…
A: Given:- We have given a draw of the system as Find:- Determine the reactions at A and B for the…
Q: Put a graph of the activity against time for a Sample for protactinium-234 use graph to find the…
A:
Q: 2. Add two vectors from your previous problem in Activity 3, A= 3.20 N at 0 and B =2.00 N at 120,…
A:
Q: Why does a chain reaction not occur in uranium mines?
A: Naturally occurring uranium has 99.3 % of U-238 and 0.7% of U-235. Only the 0.7% of U-235 is…
Q: Which particle will not be affected by the presence of an electric field? a Alpha b Beta+ c…
A: the correct solution is:d) NeutronNeutrons are electrically neutral debris, which means they haven't…
Q: Explain the reaction takes place in a perfectly sealed and insulated container, then the total…
A: Given, Reaction takes place in a perfectly sealed and insulated container.
Q: In the following reaction, which type of decay is occurring? N C + - beta plus gamma beta minus…
A: In Beta plus decay, the atomic mass of the particle remains the same, whereas the atomic number of…
Q: Which nuclide is best represented by Y? 16F → X + 1p then X → Y + e+…
A: 16F → X + 1p then X → Y + e+To find: Which nuclide is best represented by Y?
Q: What is the nuclear equation for C (beta deca а. a. 1C → 14N + ĝe b. 1C N + de -> c. C - 14N + Ge d.…
A: Answer:) option(c)
Q: 43. Complete these nuclear reactions: Li + ?→Be + ¿n 35 32 1Cl + ? → 165 + He Be + He → 3 {He + ?…
A: Given, Nuclear reactions
Q: 6. Given the information below, how much energy is emitted in the reaction? 23 22.9945 mass of Ne 1…
A:
Q: In the following reaction, which type of decay is occurring? 80 80- 35 Br 3Kr+ Obeta plus beta minus…
A: The process of spontaneous emission of an electron or a positron from the nucleus is called beta -…
Q: Question - The half-life
A: The half-life of 60Co = 5.27 year The activity of a 60Co is 3.50 x 109 Bq Find:- What is the…
Q: Please see 3c
A:
Q: What percentage of reactant will remain after 120 seconds if the half-life is 60 seconds for a…
A:
Q: Complete the following reaction 81Rb + n + ex 81 Br + Ve 81y| 81 Kr e р 81 Sr
A: A beta particle is an electron or a positron. Beta decay is a type of radioactive decay that takes…
Q: 6. Find the threshold kinetic energy for the following reactions: (a) p+p→n+E +Kº+z (b) p+n→p+E+K*
A:
Q: How many neutrons in the following element? 238 92 Select one: O a. 238 O b. 92 O c. 330 O d. 146
A: Given: The atomic mass of the element is 238. The atomic number is 92.

Step by step
Solved in 2 steps with 2 images

- 1. Calculate the Q value of the reaction: ointe) Li+He- "B+ ¿n 108 Which of the following reactions are possible or not possible, and why: (a) a +57Fe --> n +6⁰Ni; (b) p + 58Ni --> ³He +57 Co; (c) n + 157 Gd --> d + 150Eu ; Am --> a + 237Pu. 241 (d)Complete the following reaction. 1 197 4 H + ? to Au + He 1 79 2
- (a) Determine the product of the reaction Li + *He → ? + n. 3 4 2 chemPad XX → = Greek 10B5 Help 10B_5 An atomic mass is missing from your answer or could not be identified.How does a nucleus stay together despite the electrical repulsion between protons? Group of answer choices a. The negative electrons push the positive protons together b. electrical forces do not act inside a nucleus c. here are other forces in the nucleus that are stronger d. None of theseWhy do larger nuclei tend to be less stable? Group of answer choices a. Extra neutrons reduce the nuclear forces b. Coulomb forces decrease with increasing size c. Nuclear forces are very short-ranged d. None of these