Everything needed is included Consider the reversible and isothermal compression of 2.0 moles of ammonia (NH3 (g)) from an initial volume of 10.0 L to a final volume of 1.00 L, at a constant temperature of 298 K: 6a. Calculate the work w and heat q associated with this change in volume, considering NH3 to be an ideal gas. 6b. Calculate the work w (but not the heat) using the virial equation of state, with second virial coefficient B = −0.297 L/mol for NH3 at this temperature (and ignoring any higher coefficients).
Everything needed is included
Consider the reversible and isothermal compression of 2.0 moles of ammonia (NH3 (g)) from an initial volume of 10.0 L to a final volume of 1.00 L, at a constant temperature of 298 K:
6a. Calculate the work w and heat q associated with this change in volume, considering NH3 to be an ideal gas.
6b. Calculate the work w (but not the heat) using the virial equation of state, with second virial coefficient B = −0.297 L/mol for NH3 at this temperature (and ignoring any higher coefficients).

A reversible and isothermal compression process is considered. In this process, the change in internal energy is zero.
So, heat q = -w (work done)
(a) For number of moles (n) = 2, initial volume (Vi) =10L, final volume (Vf) = 1L at temperature T = 298 K, work (w) and heat (q) involved can be calculated using gas constant (R), as,
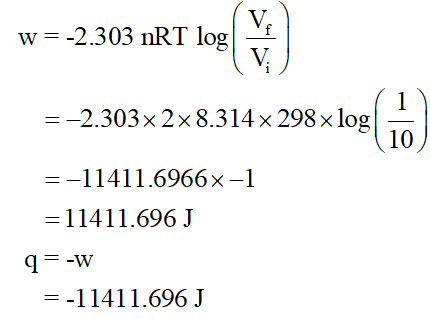
Step by step
Solved in 3 steps with 2 images









