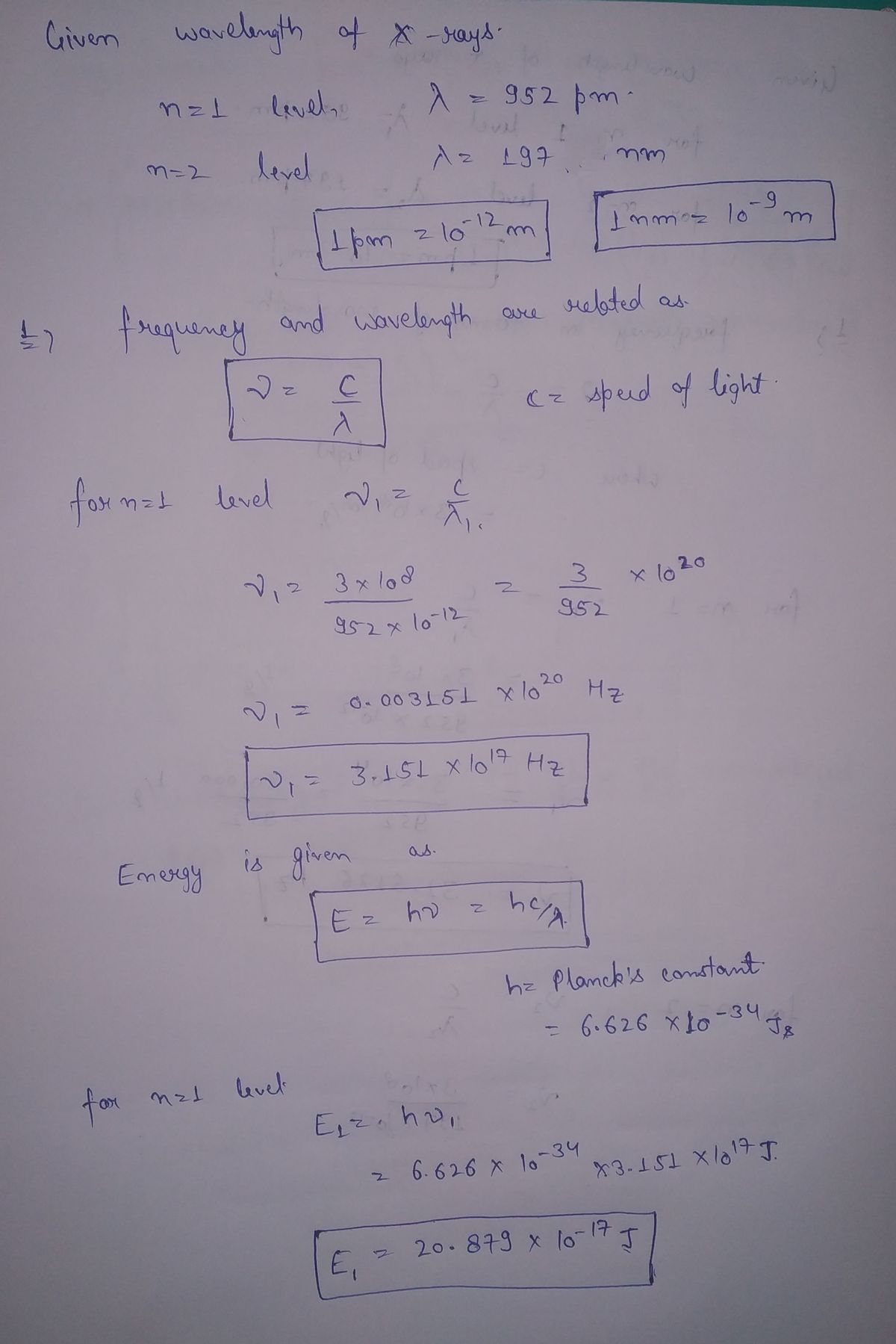
Electrons in multielectron atoms absorb X-rays at characteristic energies, leading to ionization. The characteristic energies for each element allow scientists to identify the element. For magnesium, X-rays with λ = 952 pm are required to selectively eject an electron from the n = 1 energy level, and X-rays with λ = 197 nm remove an electron from the n = 2 energy level. Calculate the frequency and energy of those X-rays. Why are the wavelengths and energies different for the two electrons.
Compton effect
The incoming photons' energy must be in the range of an X-ray frequency to generate the Compton effect. The electron does not lose enough energy that reduces the wavelength of scattered photons towards the visible spectrum. As a result, with visible lights, the Compton effect is missing.
Recoil Velocity
The amount of backward thrust or force experienced by a person when he/she shoots a gun in the forward direction is called recoil velocity. This phenomenon always follows the law of conservation of linear momentum.
Electrons in multielectron atoms absorb X-rays at characteristic energies, leading to ionization. The characteristic energies for each element allow scientists to identify the element. For magnesium, X-rays with λ = 952 pm are required to selectively eject an electron from the n = 1 energy level, and X-rays with λ = 197 nm remove an electron from the n = 2 energy level.
- Calculate the frequency and energy of those X-rays.
- Why are the wavelengths and energies different for the two electrons.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images









