E △E=2×10-21 J DE=10-21 J E2 E1 Eo
Q: Why is the entropy higher for a system with 5 particles with energy states >1 than for a system with…
A: Entropy increases precisely for energy states>1 because energy is conserved. To hold a…
Q: (c) A meteorite made of iron with mass Mmet = 100kg and initial temperature Tmet = 3000k, falls into…
A:
Q: (1) When the energy of a system (simple harmonic oscillator) is given by E = heat bath of (constant)…
A:
Q: Consider system with just two states. Take the two states to have energies 0 and ε. Partition…
A: I have written formula
Q: For an isothermal reversible expansion of two moles of an ideal gas, what is the entropy change of…
A: Number of moles: To calculate the entropy change of the gas and the surroundings during an…
Q: By considering how the total entropy changes at fixed temperature, show that particles will flowfrom…
A:
Q: T04.2 Atoms in a harmonic trap We consider Nparticles in one dimension in an external potential,…
A:
Q: Show that the following relations hold for a reversible adiabatic expansion of an ideal gas: TV-1 =…
A: According to the 1st law of thermodynamics :dQ = dU + dW. Now, for an adiabatic process, dQ = 0.So,…
Q: = T T V
A:
Q: Starting with the Clausius Inequality, ∂S ≥ ∂q/T, can you prove that, under conditions of constant…
A:
Q: Express the heat capacity of a gas at constant pressure, Cp, in terms of a partial differential…
A: Given:- we have to write the formula of heat capacity of a gas at constant pressure in terms of…
Q: Discuss the properties of three major statistics (Maxwell-Boltzmann, Fermi-Dirac, Bose-Einstein).…
A: Maxwell-Boltzmann method - According to statistics all particles of the system are assumed to be…
Q: Use statistical thermodynamic arguments to show that for a perfect gas, (∂E/∂V)T = 0.
A: Solution: Write Maxwell's thermodynamical relation given below: ∂S∂VT=∂P∂TV…
Q: Determine the amount by which the star's thermal radiation increases the entropy of the entire…
A: Entropy The entropy change of a system if ∆Q amount of heat is absorbed by the system at temperature…
Q: Consider a star that is a sphere with a radius of 6.38 108 m and an average surface temperature of…
A: Given data: Radius R =6.38×108 m Star's temperature T= 5200 K Universe's temperature T'= 2.73 K
Q: (1) When the energy of the system (simple harmonic oscillator) is given by E = (n +;) hw in a heat…
A: The partition function is given as, Z=∑n=0∞e-EnkT Thus,…
Q: lculate the work for an ideal gas that expands isothermally at T0 from V1 to V2.
A:
Q: Can you show that ∂U/∂P )T =0 J/Pa for a perfect gas? Hint: Start with ∂U = T ∂S − P ∂V. Quickly,…
A:
Q: By considering how the total entropy changes at fixed temperature, show that particles will flow…
A: To show that particles will flow from system A to system B if the chemical potentials…
Calculate the partition function for the following system at 300 K.

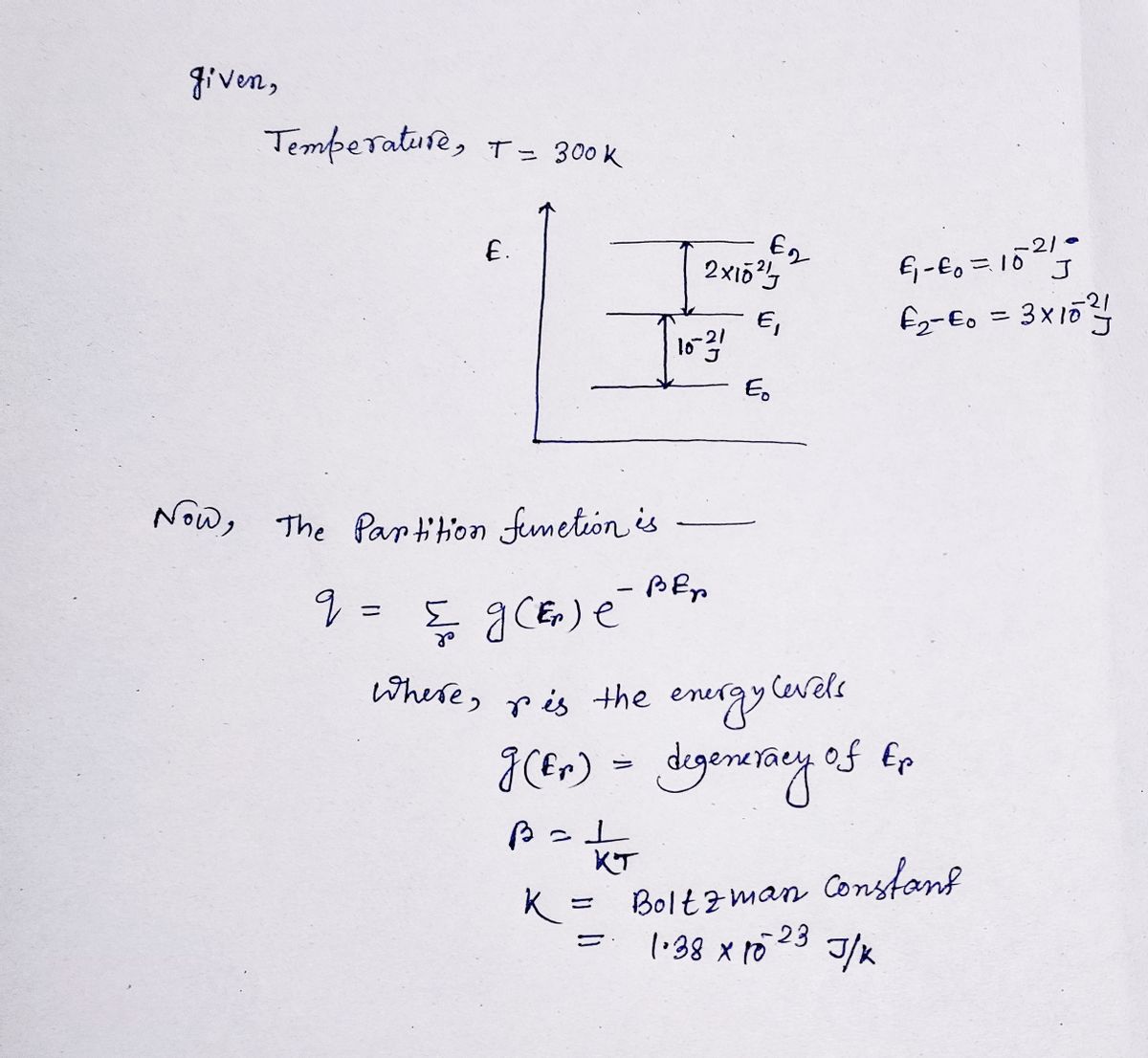
Step by step
Solved in 3 steps with 4 images

- Derive the heat capacity Cv of a system whose canonical partition function is Z = AVNT³N where A is a temperature-independent constant.Learning Goal: To derive the expression for the work done by an expanding gas, W pAV, and to understand how it follows from the expression WFAz for mechanical work In thermodynamics, positive work is defined to be the work done by a system on the exterior world. In classical mechanics, the converse is true: One always considers the work done on a system by the outside world to be positive. For example, suppose you push a large block with a certain force of Figure 101 Gas Part C What is AV, the increase in volume of the gas? Express the increase in volume in terms of Ar and other given quantities. >View Available Hint(s) - ΑΣΦ AV - PAAx Submit Previous Answers Request Answer * Incorrect; Try Again Part D Complete previous part(s) Part E Complete previous part(s) Part F Complete previous part(s) F 02-23-2For a p, v, T system formed by a mole, it has been empirically obtained that the internal energy per unit mass can be written in the form: u = apv+b, where a, b are constants. Find the equation for the adiabat in this system.