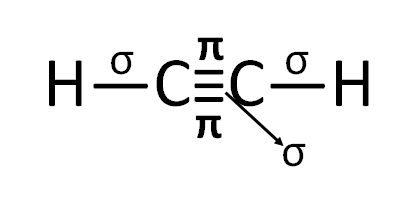
Draw the structure of Ethyne
In ethyne, there is a triple bond between two carbon atoms, and among the triple bond one is a sigma bond and the other two are pi bonds, there is also two C-H sigma bond. Both carbon atoms are sp hybridized. One sp orbital of one carbon atom overlap with another sp orbital of another carbon atom and forms a C-C sigma bond and the remaining sp orbital of both carbon atoms overlap with 1s orbital of H atom, therefore, forms two C-H sigma bond. One 2Py orbital of one carbon atom overlap with another 2Py orbital of another carbon atom and forms a pi bond, similarly, One 2Pz orbital of one carbon atom overlap with another 2Pz orbital of another carbon atom and forms a pi bond.
Therefore, the structure of ethyne is----
Step by step
Solved in 2 steps with 2 images









