Draw the general shape of a pH curve for the titration of 10 mL of 5.0 M NaNH2(NH2-is a strong base) with 2.0 M HCl(2 points). On the curve, indicate the points or regions that correspond to the following: (a)The equivalence points (give the mL of HCladded at each equivalence point) (b)Circle the buffer region(s) (c) Without doing RICE table and detailed calculation, determine whether the solution is acidic or basic at the equivalence points. Explain by identifying the major speciesand dominate chemical reaction.
Draw the general shape of a pH curve for the titration of 10 mL of 5.0 M NaNH2(NH2-is a strong base) with 2.0 M HCl(2 points). On the curve, indicate the points or regions that correspond to the following:
(a)The equivalence points (give the mL of HCladded at each equivalence point)
(b)Circle the buffer region(s)
(c) Without doing RICE table and detailed calculation, determine whether the solution is acidic or basic at the equivalence points. Explain by identifying the major speciesand dominate
(a) The given titration is acid-base titration,
The balanced chemical equation for the acid-base titration is given below,
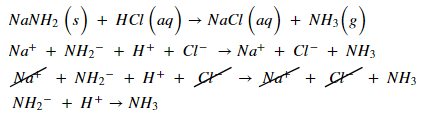
In the equivalence point, the pH = 7 (neutral).
Therefore, calculating the volume of HCl added at the equivalence point,
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









