Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

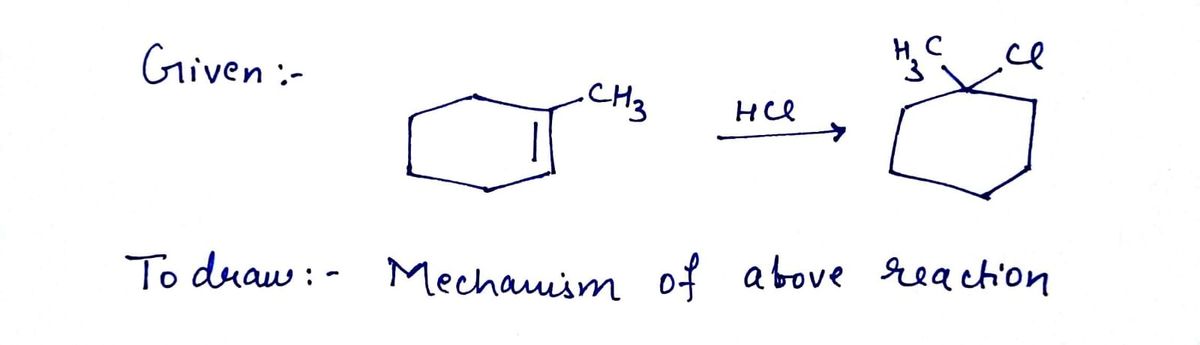
**Reaction:**
1. **Reactant:** A cyclohexene with a methyl group (CH₃) attached.
2. **Reagent:** HCl (hydrochloric acid).
3. **Product:** A chlorocyclohexane derivative with a chloride (Cl) and methyl group (CH₃) on the ring.
**Instructions:**
- Draw all missing reactants and/or products by placing atoms on the canvas and connecting them with bonds.
- Add charges where necessary.
- Use electron-flow arrows to indicate the movement of electrons. Arrows should start on the electron(s) of an atom or a bond and end on an atom, bond, or location where a new bond should be created.
**Note:**
- Click on "View Available Hint(s)" for assistance.
- Use the toolbar to adjust the structure or mechanism as needed.
**Footer:**
- Copyright © 2021 Pearson Education Inc. All rights reserved.
- Terms of Use | Privacy Policy | Permissions | Contact Us
**Additional Resources:**
- Review | Constants | Periodic Table
This exercise is aligned with organic chemistry curriculum standards, providing essential practice in understanding and illustrating reaction mechanisms.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F326fda20-abe8-40d0-9713-e7b2662a36d7%2F211b9bff-3a8b-4534-9a83-56fd479c0c0e%2F26kgl3_processed.jpeg&w=3840&q=75)
Transcribed Image Text:### Part A
**Task:**
Draw a mechanism for the following reaction.

**Reaction:**
1. **Reactant:** A cyclohexene with a methyl group (CH₃) attached.
2. **Reagent:** HCl (hydrochloric acid).
3. **Product:** A chlorocyclohexane derivative with a chloride (Cl) and methyl group (CH₃) on the ring.
**Instructions:**
- Draw all missing reactants and/or products by placing atoms on the canvas and connecting them with bonds.
- Add charges where necessary.
- Use electron-flow arrows to indicate the movement of electrons. Arrows should start on the electron(s) of an atom or a bond and end on an atom, bond, or location where a new bond should be created.
**Note:**
- Click on "View Available Hint(s)" for assistance.
- Use the toolbar to adjust the structure or mechanism as needed.
**Footer:**
- Copyright © 2021 Pearson Education Inc. All rights reserved.
- Terms of Use | Privacy Policy | Permissions | Contact Us
**Additional Resources:**
- Review | Constants | Periodic Table
This exercise is aligned with organic chemistry curriculum standards, providing essential practice in understanding and illustrating reaction mechanisms.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY