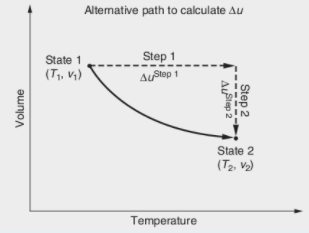
Develop a methodology (by rigorous formulation) for calculating Au according to the path shown in the Figure below, in which the change in T occurs when intermolecular interactions are important. Alternative path to calculate Au State 1 Step 1 (T1, v;) AuStep 1 State 2 (T2, V2) Temperature Step 2 Step 2 Volume
Develop a methodology (by rigorous formulation) for calculating Au according to the path shown in the Figure below, in which the change in T occurs when intermolecular interactions are important. Alternative path to calculate Au State 1 Step 1 (T1, v;) AuStep 1 State 2 (T2, V2) Temperature Step 2 Step 2 Volume
Related questions
Question

Transcribed Image Text:Develop a methodology (by rigorous formulation) for calculating Au according to the path shown in the Figure below, in which the change in T occurs when
intermolecular interactions are important.
Alternative path to calculate Au
State 1
Step 1
(T1, v1)
AuStep 1
State 2
(T2, V2)
Temperature
Step 2
AStep 2
Volume
Expert Solution
Step 1
Given:
The process is as follows:
Introduction:
A thermodynamic process taking place at constant volume is known as the isochoric process. It is also sometimes called an isometric process or constant-volume process. An isothermal process is a thermodynamic process in which the temperature of a system remains constant. The transfer of heat into or out of the system happens so slowly that thermal equilibrium is maintained.
Step by step
Solved in 2 steps with 1 images
