Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Determine the \( pH \) of each of the following solutions.**
This instruction prompts students to calculate the pH for a set of given solutions. The pH is a scale used to specify the acidity or basicity of an aqueous solution. Solutions with a pH less than 7 are acidic, while those with a pH greater than 7 are basic (alkaline); a pH of 7 is neutral. To determine the pH, students may need to apply knowledge of logarithmic calculations and possibly the use of pH formulas or concepts, such as the concentration of hydrogen ions in a solution.
There are no graphs or diagrams accompanying the text.
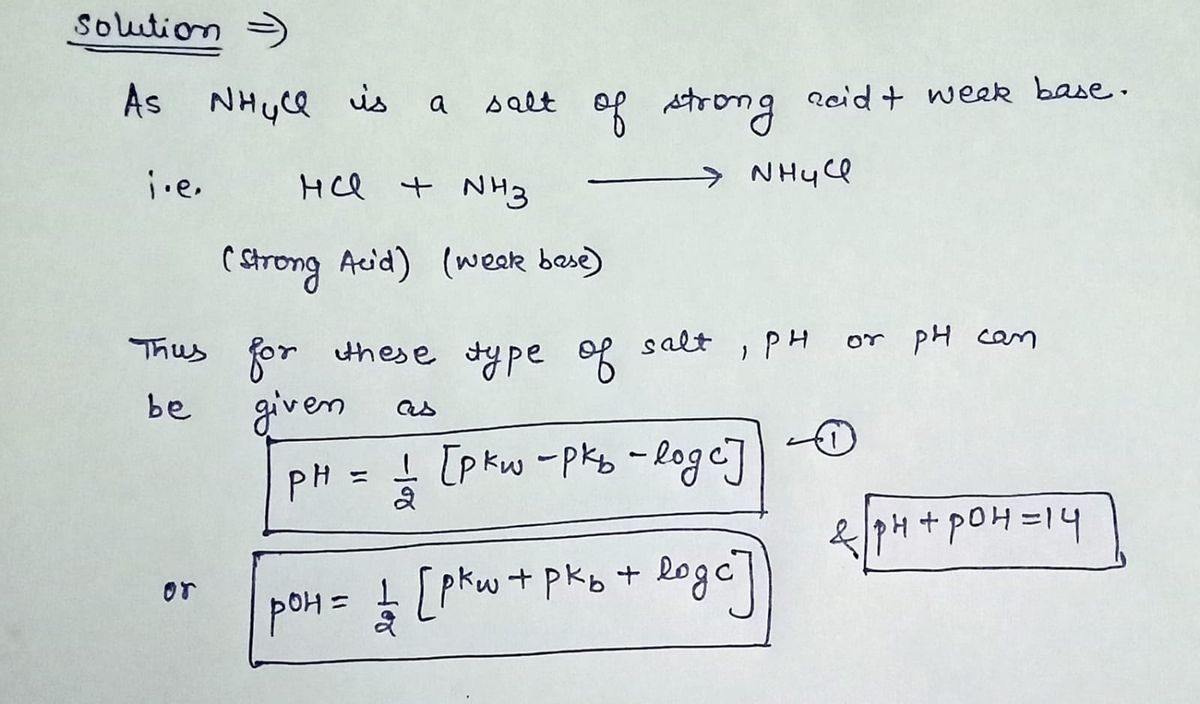
![### Calculating the pH of a 0.18 M NH₄Cl Solution
**Problem Statement:**
Determine the pH of a 0.18 M solution of NH₄Cl. Express your answer to two decimal places.
**Solution Input Box:**
A field is provided for inputting the calculated pH value. Tools for editing mathematical symbols and expressions are available, such as a square root symbol and a sigma for summation. Arrows allow undo and redo of actions, along with a help button represented by a question mark for additional assistance.
---
### Explanation:
**Key Concepts:**
1. **Ammonium Chloride (NH₄Cl):**
- NH₄Cl is a salt formed from the reaction of a strong acid (HCl) and a weak base (NH₃). When dissolved in water, it dissociates into NH₄⁺ and Cl⁻ ions.
2. **Hydrolysis of NH₄⁺:**
- The NH₄⁺ ion undergoes hydrolysis to produce H⁺ ions, which affect the pH of the solution.
3. **Calculating pH:**
- Use the equation for hydrolysis and the Kb for NH₃ to find the concentration of H⁺ ions, then apply the formula:
\[
pH = -\log[H⁺]
\]
**Steps to Solve:**
1. Write the hydrolysis equation for NH₄⁺.
2. Calculate the equilibrium concentrations using the initial concentration (0.18 M) and the Kb or Ka.
3. Solve for the H⁺ concentration.
4. Calculate the pH, ensuring the answer is to two decimal places.
For educational purposes, use these steps to practice calculating the pH for different concentrations of NH₄Cl and understand the effects of weak bases and their conjugate acids in solutions.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8bccc44d-7782-46f2-838e-c140c2dedd7b%2F0f680b42-978b-4d8c-b368-db0802c802bd%2Fawctuf_processed.png&w=3840&q=75)
Transcribed Image Text:### Calculating the pH of a 0.18 M NH₄Cl Solution
**Problem Statement:**
Determine the pH of a 0.18 M solution of NH₄Cl. Express your answer to two decimal places.
**Solution Input Box:**
A field is provided for inputting the calculated pH value. Tools for editing mathematical symbols and expressions are available, such as a square root symbol and a sigma for summation. Arrows allow undo and redo of actions, along with a help button represented by a question mark for additional assistance.
---
### Explanation:
**Key Concepts:**
1. **Ammonium Chloride (NH₄Cl):**
- NH₄Cl is a salt formed from the reaction of a strong acid (HCl) and a weak base (NH₃). When dissolved in water, it dissociates into NH₄⁺ and Cl⁻ ions.
2. **Hydrolysis of NH₄⁺:**
- The NH₄⁺ ion undergoes hydrolysis to produce H⁺ ions, which affect the pH of the solution.
3. **Calculating pH:**
- Use the equation for hydrolysis and the Kb for NH₃ to find the concentration of H⁺ ions, then apply the formula:
\[
pH = -\log[H⁺]
\]
**Steps to Solve:**
1. Write the hydrolysis equation for NH₄⁺.
2. Calculate the equilibrium concentrations using the initial concentration (0.18 M) and the Kb or Ka.
3. Solve for the H⁺ concentration.
4. Calculate the pH, ensuring the answer is to two decimal places.
For educational purposes, use these steps to practice calculating the pH for different concentrations of NH₄Cl and understand the effects of weak bases and their conjugate acids in solutions.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY