Determine the mass of each of the following:
a) 0.02 mol KOH
b) 1.500 mol CH4
The quantity obtained on dividing mass and molar weight of compounds is termed as moles. It tell us the total count of molecules present in any given mass and for 1 mole, the number of molecules is always 6.022×1023.
Given:
The number of moles of KOH is 0.02 mol.
The number of moles of CH4 is 1.500 mol.
The relation between mass and moles is shown below.
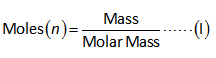
Part (a):
The molar mass of KOH is calculated as shown below.
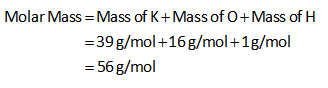
The moles of KOH are 0.02 mol.
Substitute all known values in equation (I).
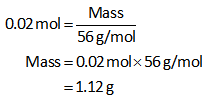
Part (b):
The molar mass of CH4 is calculated as shown below.
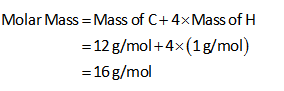
The moles of CH4 are 1.5 mol.
Substitute all known values in equation (I).
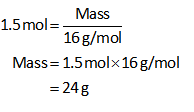
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images









