Determine the catalyst weight necessary to achieve 50% conversion when ethylene oxide is to be made by the vapor-phase catalytic oxidation of ethylene with air: C2H4 +0.502 → CH₂OCH2 A +0.5B → C Ethylene and oxygen (in air) are fed in stoichiometric proportions to a packed-bed reactor isothermally at 260°C. Ethylene is fed at a rate of 0.30 lbmol/s at a pressure of 10 atm. It is proposed to use 10 banks of 1.5 in-diameter schedule 40 tubes packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to each tube is to be 3×104 lbmol/s. The properties of the reacting fluid are to be considered identical to those of air at this temperature and pressure. The density of the catalyst particles is 120 lb/ft³ and the bed void fraction is 0.40. The rate law is - r'a = k(PA)¹/³ (PB) 2/3 lbmol/(lb cat-hr), 0.0141 lbmol/(atm·lb cat·hr) at 260°C. The pressure drop along the reactor can be with k = neglected. Note: Show the differential equation that needs to be solved. If you use MATLAB to solve the differential equation, submit your MATLAB code along with your solution.
Determine the catalyst weight necessary to achieve 50% conversion when ethylene oxide is to be made by the vapor-phase catalytic oxidation of ethylene with air: C2H4 +0.502 → CH₂OCH2 A +0.5B → C Ethylene and oxygen (in air) are fed in stoichiometric proportions to a packed-bed reactor isothermally at 260°C. Ethylene is fed at a rate of 0.30 lbmol/s at a pressure of 10 atm. It is proposed to use 10 banks of 1.5 in-diameter schedule 40 tubes packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to each tube is to be 3×104 lbmol/s. The properties of the reacting fluid are to be considered identical to those of air at this temperature and pressure. The density of the catalyst particles is 120 lb/ft³ and the bed void fraction is 0.40. The rate law is - r'a = k(PA)¹/³ (PB) 2/3 lbmol/(lb cat-hr), 0.0141 lbmol/(atm·lb cat·hr) at 260°C. The pressure drop along the reactor can be with k = neglected. Note: Show the differential equation that needs to be solved. If you use MATLAB to solve the differential equation, submit your MATLAB code along with your solution.
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Your MATLAB question does not match the subject you selected. Please ask a question in one of the 30+ subjects available. We've credited a question to your account.
Your Question:
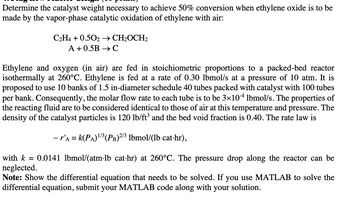
Transcribed Image Text:Determine the catalyst weight necessary to achieve 50% conversion when ethylene oxide is to be
made by the vapor-phase catalytic oxidation of ethylene with air:
C2H4 +0.502 → CH₂OCH2
A +0.5B → C
Ethylene and oxygen (in air) are fed in stoichiometric proportions to a packed-bed reactor
isothermally at 260°C. Ethylene is fed at a rate of 0.30 lbmol/s at a pressure of 10 atm. It is
proposed to use 10 banks of 1.5 in-diameter schedule 40 tubes packed with catalyst with 100 tubes
per bank. Consequently, the molar flow rate to each tube is to be 3×104 lbmol/s. The properties of
the reacting fluid are to be considered identical to those of air at this temperature and pressure. The
density of the catalyst particles is 120 lb/ft³ and the bed void fraction is 0.40. The rate law is
- r'a = k(PA)¹/³ (PB) 2/3 lbmol/(lb cat-hr),
0.0141 lbmol/(atm·lb cat·hr) at 260°C. The pressure drop along the reactor can be
with k =
neglected.
Note: Show the differential equation that needs to be solved. If you use MATLAB to solve the
differential equation, submit your MATLAB code along with your solution.
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The
