Description positively charged negatively charged no charge outside the nucleus components of the nucleus have significant mass protons Particle protons electrons neutrons electrons neutrons protons 44 electrons Answer Bank protons neutrons electrons
Electronic Transitions and Spectroscopy
The term “electronic” connotes electron, and the term “transition” implies transformation. In a molecule, the electrons move from a lower to a higher energy state due to excitation. The two energy states, the ground state and the excited state are the lowest and the highest energy states, respectively. An energy change is observed with this transition, which depicts the various data related to the molecule.
Photoelectron Spectroscopy
Photoelectron spectroscopy (PES) is a part of experimental chemistry. It is a technique used in laboratories that involves projecting intense beams of radiation on a sample element. In response, the element ejects electrons for which the relative energies are measured.

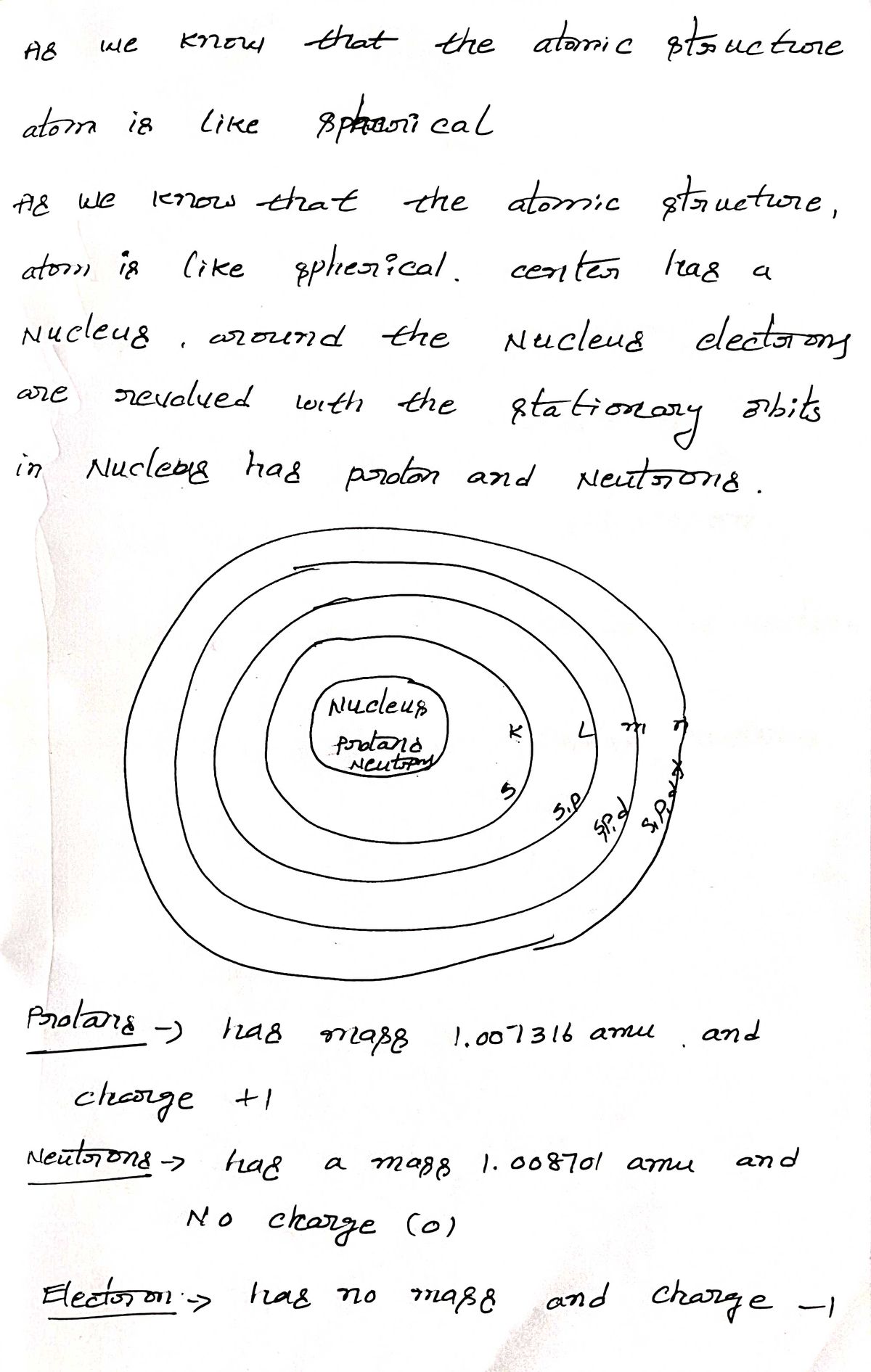
Step by step
Solved in 2 steps with 2 images









