Derive expressions for the surface-mean and mass-mean diameter from a particle-size analysis based on counting, rather than weighing, particles in given size ranges, letting Ni be the number of particles in a given size range of average diameter D-pi .
Derive expressions for the surface-mean and mass-mean diameter from a particle-size analysis based on counting, rather than weighing, particles in given size ranges, letting Ni be the number of particles in a given size range of average diameter D-pi .
Assume that all the particles are of the same shape.
Let the number of particles for the given size range be Ni and the average diameter of the particles in the given size range be D-pi.
In terms of mass fraction, the surface-mean diameter is defined as:

The relationship for the number of particles and mass fraction of the particles in a particular size range is:
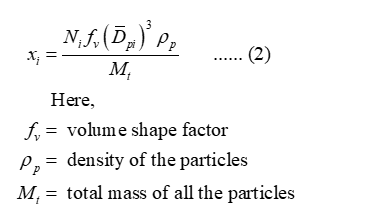
Apply summation of mass fraction for equation (2) as:
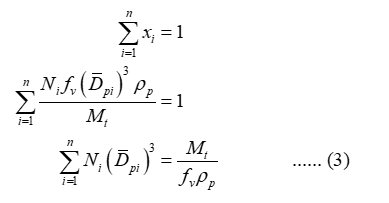
Substitute equation (2) in equation (1) so that,
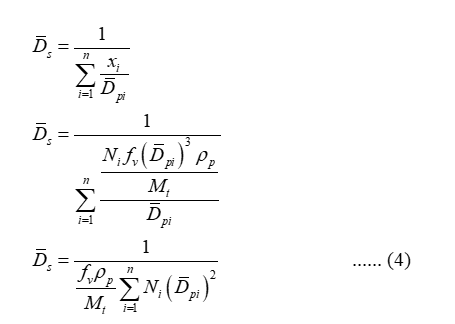
Step by step
Solved in 9 steps with 8 images









