Defined as the point at which the saturated liquid and saturated vapor states are identical, and what is called the This point.
Defined as the point at which the saturated liquid and saturated vapor states are identical, and what is called the This point.
Let us understand this by example of water:
Water is a pure substance. It means it has uniform chemical composition throughout a mixture. We draw the T-s (Temperature vs entropy) diagram of water, to know its properties.
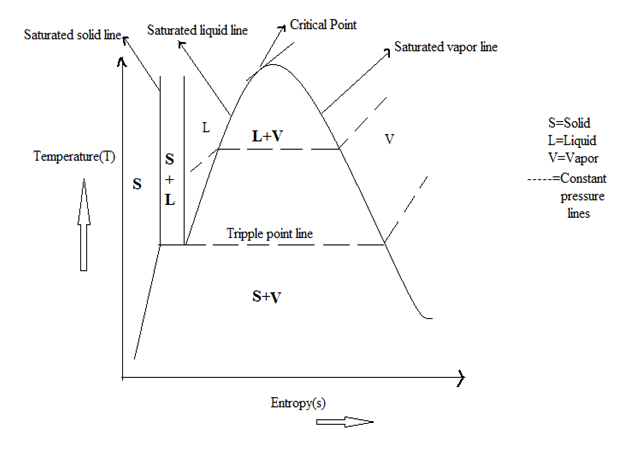
Critical point is the point with pressure and temperature where liquid and vapour state can not be distinguished, it is the end point of a phase diagram.. When water is heated below the critical pressure it first becomes a mixture of liquid and vapour and then becomes saturated vapour and then finally a superheated vapour. At critical point there is no difference saturated liquid state and saturated vapour state. There is no change of liquid water into vapour state form above critical point.
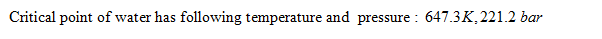
Step by step
Solved in 3 steps with 2 images









