CovalentActivity.do?locator3Dassignment-take [Review Topics] [References] Use the References to access important values if needed for this question. Br + 6NO3 + 6H →BR03 + 6NO2+ 3H20 In the above reaction, the oxidation state of nitrogen changes from -3 7 to +2 How many electrons are transferred in the reaction? 2 Submit Answer Retry Entire Group 9 more group attempts remaining
CovalentActivity.do?locator3Dassignment-take [Review Topics] [References] Use the References to access important values if needed for this question. Br + 6NO3 + 6H →BR03 + 6NO2+ 3H20 In the above reaction, the oxidation state of nitrogen changes from -3 7 to +2 How many electrons are transferred in the reaction? 2 Submit Answer Retry Entire Group 9 more group attempts remaining
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![CovalentActivity.do?locator3Dassignment-take
[Review Topics]
[References]
Use the References to access important values if needed for this question.
Br + 6NO3 + 6H
→BR03 + 6NO2+ 3H20
In the above reaction, the oxidation state of nitrogen changes from -3
7 to +2
How many electrons are transferred in the reaction? 2
Submit Answer
Retry Entire Group
9 more group attempts remaining
<Previous
4+
wwert
144](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F16cbf833-fdf3-4751-9327-e0e0ad6bfb01%2F92b230c9-2828-4a1f-a7d3-7c87c7431210%2Fg2zlok8.jpeg&w=3840&q=75)
Transcribed Image Text:CovalentActivity.do?locator3Dassignment-take
[Review Topics]
[References]
Use the References to access important values if needed for this question.
Br + 6NO3 + 6H
→BR03 + 6NO2+ 3H20
In the above reaction, the oxidation state of nitrogen changes from -3
7 to +2
How many electrons are transferred in the reaction? 2
Submit Answer
Retry Entire Group
9 more group attempts remaining
<Previous
4+
wwert
144
Expert Solution
Step 1
Given reaction,
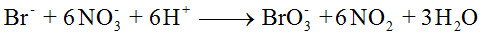
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY