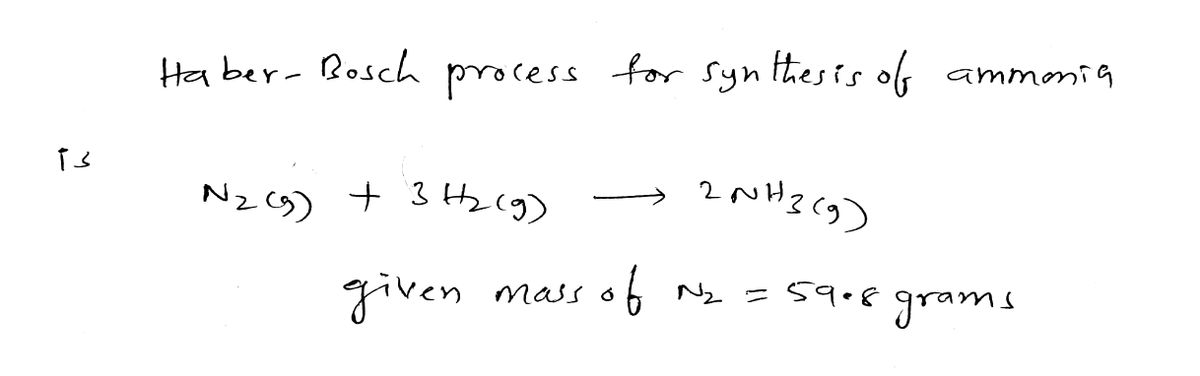
Consider the Haber-Bosch process for the synthesis of ammonia from its elements. Calculate the theoretical yield in moles NHs from the complete reaction of 59.8 grams N2 in the presence of excess H2 gas according to the following balanced chemical equation: N:(g) + 3 H2(g) → 2 NH:(g) 1 meHNE 2 mol NH3 59.8 1 mol NH3 28.02 3 metN. STARTING AMOUNT 1 mol N2 2 mol NH: 59.8 g N2 x = 1 mol NH3 28.02 g N2 3 mol N2 ARR FACTOR DELETE ANSWER RESET
Classes Of Functional Groups
Organic Chemistry deals mostly with carbon and hydrogens, also called hydrocarbons, but those groups which replace hydrogen and bonds with carbon to give a characteristic nature, unique of their own, to the hydrocarbon they are attached to, are called functional groups. All the compounds belonging to a functional group undergo reactions in a similar pattern and are known to have similar physical and chemical properties.
Characteristics Of Functional Groups
In organic chemistry, we encounter a number of special substituent groups which are attached to the hydrocarbon backbone. These groups impart certain characteristics to the molecule of which it is a part of and thus, become the highlight of that particular molecule.
IUPAC Nomenclature
In Chemistry, IUPAC stands for International Union of Pure and Applied Chemistry which suggested a systematic naming approach for the organic and inorganic compounds, as in the beginning stage of nomenclature one single chemical compound was named in many ways by which lead to confusion. The need for this approach aroused as the number of chemical compounds newly discovered were increasing (approximately 32 million compounds) and the basic concept of nomenclature i.e. the trivial nomenclature and the derived system of nomenclature failed to overcome the challenge. It is an important task to name a chemical compound systematically and unambiguously which reduces lots of confusion about the newly reported compounds.
Im havin g toublr understanding what I did wrong on this conversion, can someone write it out and walk through it like in the question?

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









