Consider the following equilibrium between bromine pentafluoride, bromine, and fluorine at 1227oC 2 B r F ( g ) B r ( g ) 5 F ( g ) 522 Write the equilibrium constant expression, Kc for the equilibrium above. 2.00 mol of Bromine gas and 1.00 mol of fluorine gas are placed in a sealed container in a 5.00 L container at 1227oC and the system is allowed to come to equilibrium. Show a complete equilibrium table expressing the equilibrium pressures of all components in terms of a variable x. 2 B r F ( g ) B r ( g ) 5 F ( g ) 522 I C E After the system has reached equilibrium the concentration of F2
Consider the following equilibrium between bromine pentafluoride, bromine, and fluorine at 1227oC
2 B r F ( g ) B r ( g ) 5 F ( g ) 522
-
Write the equilibrium constant expression, Kc for the equilibrium above.
-
2.00 mol of Bromine gas and 1.00 mol of fluorine gas are placed in a sealed container in a 5.00 L container at 1227oC and the system is allowed to come to equilibrium. Show a complete equilibrium table expressing the equilibrium pressures of all components in terms of a variable x.
2 B r F ( g ) B r ( g ) 5 F ( g ) 522
I C E
-
After the system has reached equilibrium the concentration of F2 is found to be 0.00840 mol/L. Calculate the value of the equilibrium constant Kc for the reaction at 1227oC.
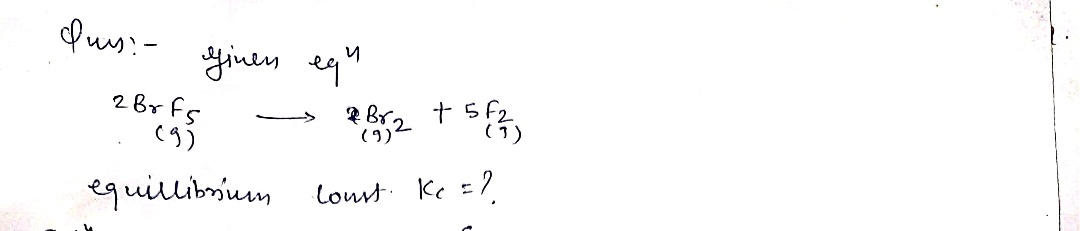
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









