Consider a water-to-water counterflow heat exchanger with these specifications. Hot water enters at 70°C while cold water enters at 20°C. The exit temperature of the hot water is 15°C greater than that of the cold water, and the mass flow rate of the hot water is 50 percent greater than that of the cold water. The product of heat transfer surface area and the overall heat transfer coefficient is 2200 W/K. Take the specific heat of both cold and hot water to be cp= 4180 J/kg.°C. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Cold water 20°C Hot water O °C Th. in Determine the mass flow rate of the cold water. The mass flow rate of the cold water is kg/s.
Consider a water-to-water counterflow heat exchanger with these specifications. Hot water enters at 70°C while cold water enters at 20°C. The exit temperature of the hot water is 15°C greater than that of the cold water, and the mass flow rate of the hot water is 50 percent greater than that of the cold water. The product of heat transfer surface area and the overall heat transfer coefficient is 2200 W/K. Take the specific heat of both cold and hot water to be cp= 4180 J/kg.°C. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Cold water 20°C Hot water O °C Th. in Determine the mass flow rate of the cold water. The mass flow rate of the cold water is kg/s.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Concept explainers
Heat Exchangers
Heat exchangers are the types of equipment that are primarily employed to transfer the thermal energy from one fluid to another, provided that one of the fluids should be at a higher thermal energy content than the other fluid.
Heat Exchanger
The heat exchanger is a combination of two words ''Heat'' and ''Exchanger''. It is a mechanical device that is used to exchange heat energy between two fluids.
Question
100%
Thank You! I have a follow up question and was wondering if you could answer it. It is shown below:

Transcribed Image Text:Required information
Consider a water-to-water counterflow heat exchanger with these specifications. Hot water enters at 70°C while cold
water enters at 20°C. The exit temperature of the hot water is 15°C greater than that of the cold water, and the mass flow
rate of the hot water is 50 percent greater than that of the cold water. The product of heat transfer surface area and the
overall heat transfer coefficient is 2200 W/K. Take the specific heat of both cold and hot water to be cp= 4180 J/kg.°C.
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
Cold water
20°C
Hot water
O
Th, in
Determine the mass flow rate of the cold water.
The mass flow rate of the cold water is
kg/s.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Thank you so much! I have one last follow up question. It is about heat transfer rate. Can you answer it too? It is shown in the screenshot:
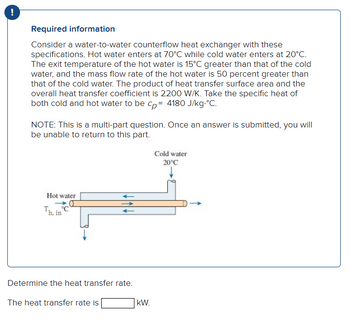
Transcribed Image Text:!
Required information
Consider a water-to-water counterflow heat exchanger with these
specifications. Hot water enters at 70°C while cold water enters at 20°C.
The exit temperature of the hot water is 15°C greater than that of the cold
water, and the mass flow rate of the hot water is 50 percent greater than
that of the cold water. The product of heat transfer surface area and the
overall heat transfer coefficient is 2200 W/K. Take the specific heat of
both cold and hot water to be cp= 4180 J/kg.°C.
NOTE: This is a multi-part question. Once an answer is submitted, you will
be unable to return to this part.
Hot water
C
Th, in
Determine the heat transfer rate.
The heat transfer rate is
kW.
Cold water
20°C
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY