Consider a proton (m = 1.6726219 x 10-27 kg) confined in a box of length 10.000 pm. What is the energy difference between the n = 1 and n = 2 states of the proton-in-a-box? Report your answer in units of 10-18 J (that is, if the energy is 5.00 × 10-18 J, you would report it as "5.00").
Consider a proton (m = 1.6726219 x 10-27 kg) confined in a box of length 10.000 pm. What is the energy difference between the n = 1 and n = 2 states of the proton-in-a-box? Report your answer in units of 10-18 J (that is, if the energy is 5.00 × 10-18 J, you would report it as "5.00").
Related questions
Question

Transcribed Image Text:Consider a proton (m = 1.6726219 × 10-27 kg) confined in a box of length 10.000 pm. What is the energy difference
between the n = 1 and n = 2 states of the proton-in-a-box?
Report your answer in units of 10-18 J (that is, if the energy is 5.00 × 10-18 J, you would report it as "5.00").
Expert Solution
Step 1
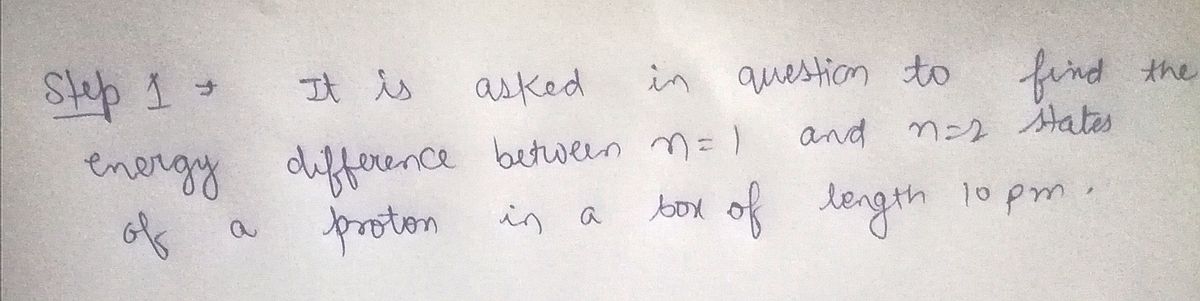
Step by step
Solved in 2 steps with 2 images
