Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
please answer them correctly, thanks

Transcribed Image Text:**Complete the following reaction and write the IUPAC names of the two products.**
**Reaction:**
The reactant is an amide:
- Structure: CH₃CH₂NHC(=O)H
- Reacts with: NaOH (in the presence of water and heat)
**Products:**
- In a typical reaction where an amide is treated with a strong base (NaOH) and heat, the products are a carboxylate salt and an amine.
**IUPAC Naming of Products:**
1. **Carboxylate Salt**: Formed from the carboxylic acid part of the amide.
2. **Amine**: Formed from the amine part of the amide.
Please fill in the IUPAC names of these products in the boxes provided.
![**Exercise Title: Naming Salts in Organic Chemistry**
**Instructions:**
Name each of the following salts.
1. **Compound:** \((\text{CH}_3\text{CH}_2)_2\text{NH}_2^+\) \(\text{CH}_3\text{COO}^-\)
**Answer Box:** [__________________]
2. **Compound:**
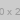
\(\text{Br}^-\)
**Answer Box:** [__________________]
**Hints:**
Below are some common amines and their structures:
- **NH\(_3\)** : Ammonia

- **Benzylamine**

- **Piperidine**

- **Aniline**

- **Pyridine**

**Diagram Descriptions:**
- The first compound contains diethylammonium and acetate ions.
- The second compound features a pyridinium ion with an ethyl group attached to it, paired with a bromide ion.
Use the given structures for reference to identify and name the compounds accurately.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F65ba74c8-a7d5-492d-89b1-b2745199e40b%2Fbdee42bd-ae0a-49fc-aa94-61378793dc42%2Fr6oe4bg_processed.png&w=3840&q=75)
Transcribed Image Text:**Exercise Title: Naming Salts in Organic Chemistry**
**Instructions:**
Name each of the following salts.
1. **Compound:** \((\text{CH}_3\text{CH}_2)_2\text{NH}_2^+\) \(\text{CH}_3\text{COO}^-\)
**Answer Box:** [__________________]
2. **Compound:**
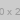
\(\text{Br}^-\)
**Answer Box:** [__________________]
**Hints:**
Below are some common amines and their structures:
- **NH\(_3\)** : Ammonia

- **Benzylamine**

- **Piperidine**

- **Aniline**

- **Pyridine**

**Diagram Descriptions:**
- The first compound contains diethylammonium and acetate ions.
- The second compound features a pyridinium ion with an ethyl group attached to it, paired with a bromide ion.
Use the given structures for reference to identify and name the compounds accurately.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY