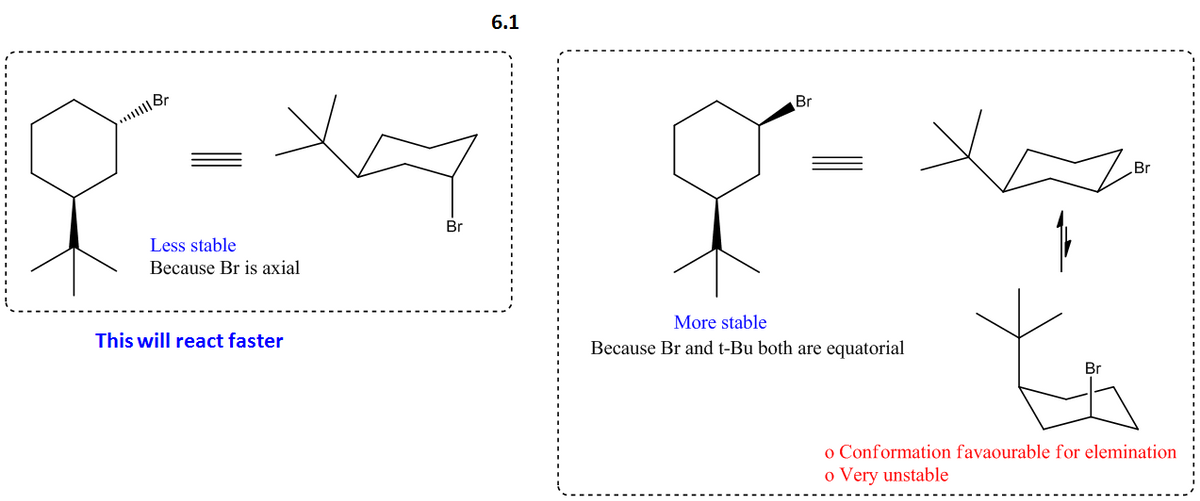
Circle the compound below, which undergoes elimination reactions much faster than the other one (in the presence of an appropriate base). 6. Br Br Draw a conformation of the faster reacting isomer which resembles the transition state. (you need to draw the cyclohexane in its chair form showing equatorial and axial orientations of the relevant bonds) 6.1. Use curly arrows on the structure you have drawn for 6.1 to show the formation of the elimination product. Use a base of your choice. Draw the structure of the elimination product. (Note that two regioisomeric elimination products are possible) 6.2. Give a brief narrative explanation for your choice of the stereoisomer, based on your illustrations in 6.1 and 6.2. 6.3.
Reactions of Ethers
Ethers (R-O-R’) are compounds formed by replacing hydrogen atoms of an alcohol (R-OH compound) or a phenol (C6H5OH) by an aryl/ acyl group (functional group after removing single hydrogen from an aromatic ring). In this section, reaction, preparation and behavior of ethers are discussed in the context of organic chemistry.
Epoxides
Epoxides are a special class of cyclic ethers which are an important functional group in organic chemistry and generate reactive centers due to their unusual high reactivity. Due to their high reactivity, epoxides are considered to be toxic and mutagenic.
Williamson Ether Synthesis
An organic reaction in which an organohalide and a deprotonated alcohol forms ether is known as Williamson ether synthesis. Alexander Williamson developed the Williamson ether synthesis in 1850. The formation of ether in this synthesis is an SN2 reaction.
![**6.** Circle the compound below, which undergoes elimination reactions much faster than the other one (in the presence of an appropriate base).
[Image Description: Two chemical structures are shown. Both are cyclohexane rings with a bromine (Br) substituent. The left structure features the Br on a secondary carbon, while the right structure has the Br on a primary carbon.]
**6.1.** Draw a conformation of the faster reacting isomer which resembles the transition state. (You need to draw the cyclohexane in its chair form showing equatorial and axial orientations of the relevant bonds.)
**6.2.** Use curly arrows on the structure you have drawn for 6.1 to show the formation of the elimination product. Use a base of your choice. Draw the structure of the elimination product.
(Note that two regioisomeric elimination products are possible.)
**6.3.** Give a brief narrative explanation for your choice of the stereoisomer, based on your illustrations in 6.1 and 6.2.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7d6a0751-6896-496c-9850-d9c5dd8d9dc3%2Fb7e8e6ee-c045-4430-8113-a7fff2bec69a%2Fg025bde_processed.jpeg&w=3840&q=75)
Step by step
Solved in 3 steps with 3 images









