Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
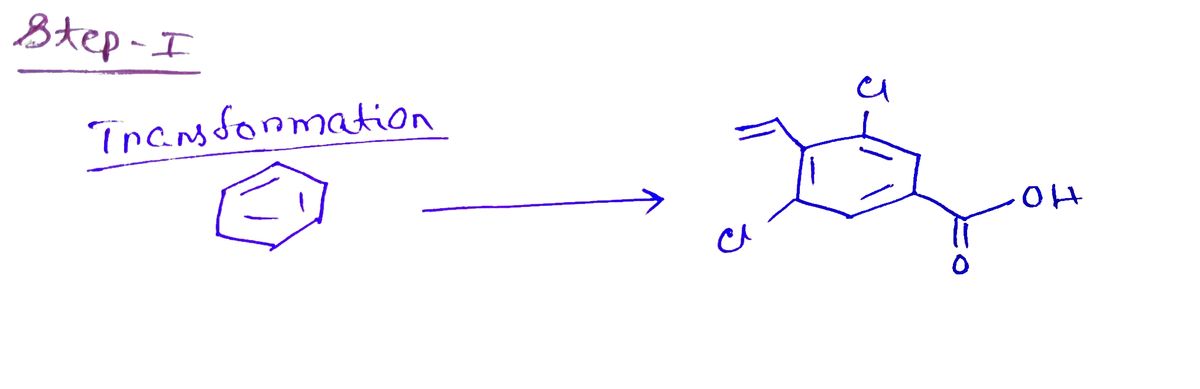
Starting from benzene, provide a synthesis of the following compound. Ensure that the product is prepared in the highest percent yield possible.
Provide all necessary reagents and draw the compound formed after each step. You do not have to use curved arrows.

Transcribed Image Text:The image displays the chemical structure of 4-chloro-3-carboxyphenyl prop-2-enoic acid. This compound features a benzene ring with the following characteristics:
1. **Benzene Ring**: At the core of the structure, there is a hexagonal benzene ring representing six carbon atoms with alternating double bonds, which is typical for aromatic compounds.
2. **Chlorine Substituents**: Two chlorine atoms (Cl) are attached to the benzene ring:
- One chlorine is at the para position (position 1) relative to the prop-2-enoic acid group.
- The other chlorine is at the meta position (position 5) from the prop-2-enoic acid group.
3. **Carboxyl Group**: At position 3, a carboxyl group (-COOH) is attached to the benzene ring. This group includes a carbon double-bonded to an oxygen atom and single-bonded to a hydroxyl group (OH).
4. **Prop-2-enoic Acid Side Chain**: Extending from the benzene ring (at position 6), there is an unsaturated side chain with one double bond, consisting of three carbon atoms. This includes a carbonyl group (C=O) adjacent to the carboxyl group.
This structure illustrates a type of chlorophenyl acrylic acid derivative, often significant in organic chemistry contexts related to synthetic and pharmaceutical applications.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY