
Monosaccharides are simplest form of sugar and the most basic units of carbohydrates that can not be hydrolyzed.
Disaccharides are the carbohydrates which are formed by joining two monosaccharides by glycosidic linkage.
Polysaccharides are the most abundant carbohydrates whose molecule contain number of sugar molecules bonded.
a) Chitin
The structure for the chitin molecule is:
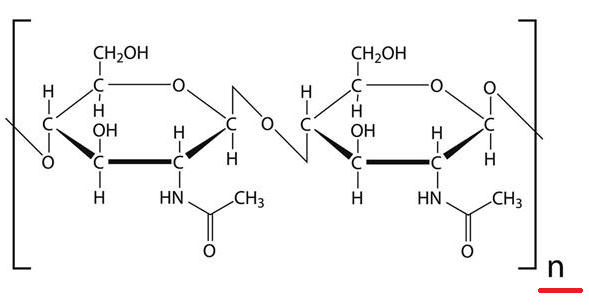
In the above structure 'n' represents number of monosaccharide units, that is chitin is the "polysaccharide".
b) Mannose
The structure for the mannose molecule is:
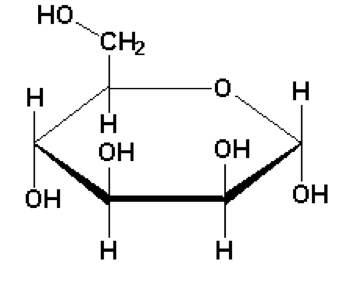
Mannose is the carbohydrate having the single unit of sugar thus mannose is a "Monosaccharide".
c) Lactose
The structure for lactose molecule is,
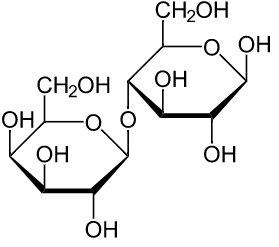
In the above structure of lactose have two units of monosaccharides joined by the glycocidic linkage thus Lactose is a"disaccharide".
Step by step
Solved in 2 steps with 6 images









