
Spectroscopy analysis is useful for the determination of functional groups and the structure of organic compounds.
Given:
The compound 42 is a liquid, which has boiling point of 81 oC. The reaction of this compound and bromine in the presence of CCl4 produces 1,1,2,2-tetrabromide. The percentage of C and H in the compound is 87.73 and 12.27 respectively.
To find the molecular formula of the compound as follows,
Percent of C in compound = 87.73%
Percent of H in compound = 12.27%
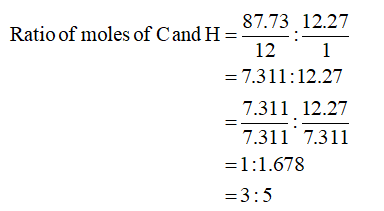
Hence, the empirical formula of the given compound is C3H5.
From the mass spectra, the molecular ion peak is seen at 82, therefore, the molecular weight of compound is 82.
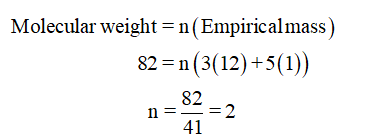
Therefore, the molecular formula of given compound is C6H10.
Calculating the Double bond equivalent (DBE) as follows,
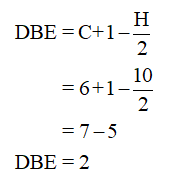
The compound reacts with two molecules of bromine in the presence of CCl4 to form 1,1,2,2-tetrabromide, hence the compound should have a triple bond.
Step by step
Solved in 7 steps with 6 images









