
Redox reactions are those in which oxidation and reduction both take place simultaneously.
The reduction is a process in which a species accepts electrons.
Oxidation is a process in which a species donates electrons.
For the whole redox reaction, the oxidation and reduction half-reaction can be written and solved by following some points.
The following steps are used to balance the half reduction reaction:
1. The oxidation number is assigned to all the elements present in the reaction
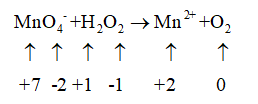
2. The reduction half-reaction is identified and written separately.
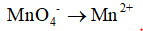
3. The elements except hydrogen and oxygen can be balanced. But here it is already balanced. So this step is skipped.
4. Oxygens are balanced by adding extra water molecules where the oxygen are less
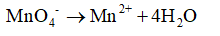
5. Hydrogens are balanced by adding hydrogen ions to the side where hydrogen is less
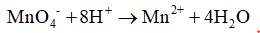
This is also the basis of the acidic condition used in the reaction.
6. The total charge is calculated on both sides and then the charge is balanced by adding electrons on the side where it is required.
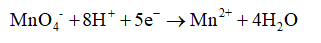
Step by step
Solved in 3 steps with 6 images









