
Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and specify the other subparts (up to 3) you’d like answered.
Redox reactions involve the transfer of electrons among the reactants in the given chemical reaction. These reactions are chemical reactions. It is an oxidation-reduction reaction and the oxidation number of molecules varies by the gain or loss of an electron.
Given:
The given half-reactions are as follows,
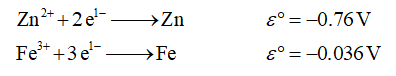
i.
To write the balanced half-reaction for the oxidation as follows,
Oxidation reaction involves the loss of electron resulting in increase of oxidation number.
The oxidation reaction is as follows,
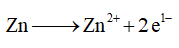
Therefore, the balanced half-reaction for the oxidation is given below,
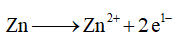
Step by step
Solved in 5 steps with 7 images









