
CH3Cl reacts with Cl2 gas to form CH2Cl2 and HCl gas. The equation for the balanced chemical reaction is given below:
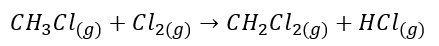
The stoichiometry of the balanced chemical reaction states that one mole of Cl2 forms one mole of HCl gas. Hence the mole of chlorine gas is equal to the moles of HCl gas.
So we can calculate the volume of the HCl gas using the gas law.
For the phase change of the ideal gas the temperature, pressure, and volume of the initial state and the final state are given by the following equation:
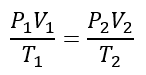
Where
P1 = initial pressure of gas (Cl2 gas)= 0.718 atm
V1 = initial volume of gas (Cl2 gas)= 3.35 L
T1 = initial temperature of gas (Cl2 gas)= 127oC = 127oC +273.15 = 400.15 K
P2 = final pressure of gas (HCl gas)= 0.430 atm
V2 = final volume of gas (HCl gas) =?
T2 = final temperature of gas (HCl gas)= 72oC = 72oC +273.15 = 345.15 K
Step by step
Solved in 4 steps with 4 images









