**Combustion Analysis of an Organic Compound** A 7.478 gram sample of an organic compound containing carbon (C), hydrogen (H), and oxygen (O) is analyzed through combustion analysis. The process produces 19.34 grams of carbon dioxide (CO₂) and 3.959 grams of water (H₂O). In a separate experiment, the molar mass is determined to be 136.2 g/mol. Use this information to determine the empirical formula and the molecular formula of the organic compound. **Enter the elements in the order C, H, O:** - Empirical formula = [____] - Molecular formula = [____] --- **Diagram Explanation:** The diagram depicted is a combustion analysis apparatus. It includes: - **Furnace:** The main unit where the sample is heated. - **Sample (labelled):** The organic compound under analysis. - **H₂O Absorber:** A unit that captures the water produced during combustion. - **CO₂ Absorber:** A unit that captures the carbon dioxide produced during combustion. These components work together to analyze the combustion of an organic compound, allowing for the measurement of CO₂ and H₂O produced, which are critical for determining the composition of the compound.
**Combustion Analysis of an Organic Compound** A 7.478 gram sample of an organic compound containing carbon (C), hydrogen (H), and oxygen (O) is analyzed through combustion analysis. The process produces 19.34 grams of carbon dioxide (CO₂) and 3.959 grams of water (H₂O). In a separate experiment, the molar mass is determined to be 136.2 g/mol. Use this information to determine the empirical formula and the molecular formula of the organic compound. **Enter the elements in the order C, H, O:** - Empirical formula = [____] - Molecular formula = [____] --- **Diagram Explanation:** The diagram depicted is a combustion analysis apparatus. It includes: - **Furnace:** The main unit where the sample is heated. - **Sample (labelled):** The organic compound under analysis. - **H₂O Absorber:** A unit that captures the water produced during combustion. - **CO₂ Absorber:** A unit that captures the carbon dioxide produced during combustion. These components work together to analyze the combustion of an organic compound, allowing for the measurement of CO₂ and H₂O produced, which are critical for determining the composition of the compound.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Combustion Analysis of an Organic Compound**
A 7.478 gram sample of an organic compound containing carbon (C), hydrogen (H), and oxygen (O) is analyzed through combustion analysis. The process produces 19.34 grams of carbon dioxide (CO₂) and 3.959 grams of water (H₂O).
In a separate experiment, the molar mass is determined to be 136.2 g/mol. Use this information to determine the empirical formula and the molecular formula of the organic compound.
**Enter the elements in the order C, H, O:**
- Empirical formula = [____]
- Molecular formula = [____]
---
**Diagram Explanation:**
The diagram depicted is a combustion analysis apparatus. It includes:
- **Furnace:** The main unit where the sample is heated.
- **Sample (labelled):** The organic compound under analysis.
- **H₂O Absorber:** A unit that captures the water produced during combustion.
- **CO₂ Absorber:** A unit that captures the carbon dioxide produced during combustion.
These components work together to analyze the combustion of an organic compound, allowing for the measurement of CO₂ and H₂O produced, which are critical for determining the composition of the compound.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F144ac4d4-58bf-4a39-b1bc-5bb4c77f8379%2F3ef005d8-ced5-4554-82d7-144ba86adfb1%2Famxkix.jpeg&w=3840&q=75)
Transcribed Image Text:**Combustion Analysis of an Organic Compound**
A 7.478 gram sample of an organic compound containing carbon (C), hydrogen (H), and oxygen (O) is analyzed through combustion analysis. The process produces 19.34 grams of carbon dioxide (CO₂) and 3.959 grams of water (H₂O).
In a separate experiment, the molar mass is determined to be 136.2 g/mol. Use this information to determine the empirical formula and the molecular formula of the organic compound.
**Enter the elements in the order C, H, O:**
- Empirical formula = [____]
- Molecular formula = [____]
---
**Diagram Explanation:**
The diagram depicted is a combustion analysis apparatus. It includes:
- **Furnace:** The main unit where the sample is heated.
- **Sample (labelled):** The organic compound under analysis.
- **H₂O Absorber:** A unit that captures the water produced during combustion.
- **CO₂ Absorber:** A unit that captures the carbon dioxide produced during combustion.
These components work together to analyze the combustion of an organic compound, allowing for the measurement of CO₂ and H₂O produced, which are critical for determining the composition of the compound.
Expert Solution
Step 1
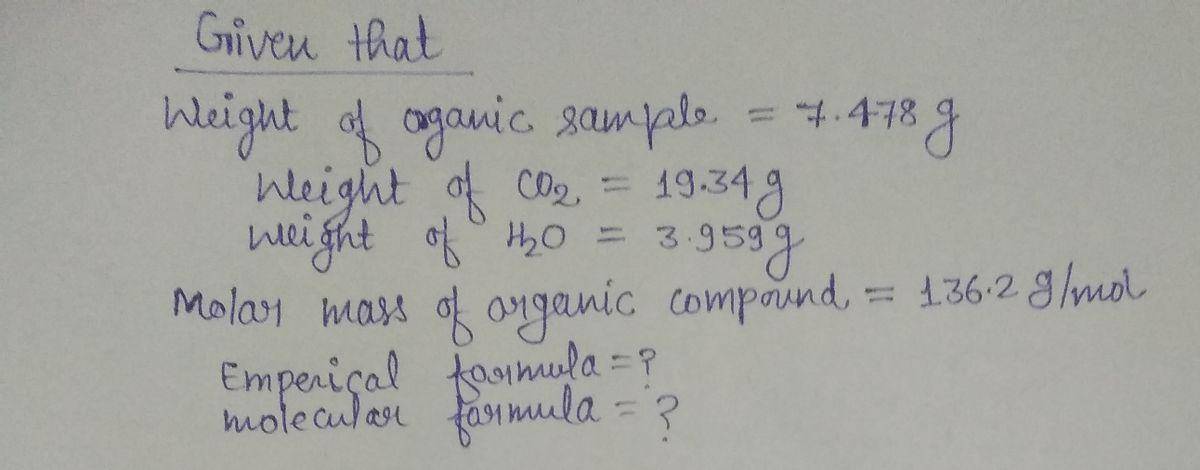
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY