
The formation of methane molecule can be explained as follows:
Electronic configuration of carbon is =1s2, 2s2, 2p2
Electronic configuration of hydrogen is = 1s1
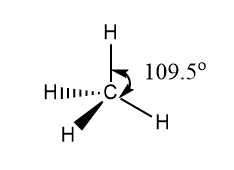
A carbon atom has four valence electrons in the outermost shell. The four atomic orbitals of carbon atom one s and three p orbitals hybridized to form four sp3 hybridized orbital. Four sp3 hybridized orbitals contain four unpaired electrons and these four electrons pairs with four 1s orbitals of four hydrogen atoms form four sigma bond.
According to the VSEPR theory four bonding orbitals arranged in tetrahedral geometry and the bond angle is 105.5o.
C-H bonding orbital has a cylindrical geometry and C-H antibonding orbital has also cylindrical geometry.
Step by step
Solved in 2 steps with 1 images









